Hyalinizing Trabecular Tumor: A Case Series with Literature Review
Abstract
Introduction
Hyalinizing trabecular tumor (HTT) is a rare thyroid neoplasm originating from follicular cells and poses diagnostic challenges due to its cytologic and histologic overlap with other thyroid malignancies. This study aims to present the clinical features and management of HTT cases treated at a single center.
Methods
This was a single-center retrospective case series. The patients were included from January 2019 to November 2024. Data collection took place over one month, from November 15, 2024, to December 15, 2024. The study included patients with HTT whose diagnoses were confirmed histopathologically.
Results
The case series included 11 patients, predominantly female, 10 (90.9%), with a mean age of 50.7±19.01 years. The most common presenting symptom was anterior neck swelling, recorded in 5 (45.5%), while one case (9.1%) was discovered incidentally. Hyperthyroidism was present in 6 (54.5%). The tumors were distributed within the thyroid gland as follows: left lobe in 5 (45.5%) cases, right lobe in 4 (36.4%) cases, and isthmus in 2 (18.1%) cases. Total thyroidectomy was performed in 7 patients (63.6%), with tumor sizes ranging from 0.5 to 5.5 cm and a mean diameter of 2.6 ± 2.05 cm. All diagnoses were confirmed postoperatively through histopathological examination.
Conclusion
A rare benign tumor, HTT remains challenging to diagnose accurately. Both total thyroidectomy and lobectomy may result in good outcomes.
Introduction
Thyroid neoplasms include a wide range of lesions with varying behavior and prognosis [1]. They are generally classified as benign, low-grade malignant, or malignant. Benign tumors, such as adenomas, are common and usually do not cause symptoms. Low-grade malignant neoplasms, like follicular thyroid carcinoma, tend to be more aggressive but often have a good prognosis [2]. Hyalinizing trabecular tumor (HTT) of the thyroid was first identified in 1987. It accounts for approximately 1% of all thyroid tumors, occurs six times more often in men than women, and is most frequently diagnosed in individuals in their 50s [3]. HTT was originally classified as a variant of follicular adenoma but is frequently misdiagnosed due to overlapping morphological features with several thyroid neoplasms. These include papillary thyroid carcinoma (PTC) and medullary thyroid carcinoma. Diagnostic challenges also extend to rare tumors with trabecular architecture, such as fetal-type follicular adenoma, poorly differentiated carcinoma, intrathyroid parathyroid neoplasms, and metastatic lesions to the thyroid [4,5]. This poses a challenge in the clinical management of these lesions, as an accurate presurgical diagnosis of HTT is essential to prevent unnecessary overtreatment of this tumor. A literature review reveals that an accurate preoperative cytological diagnosis of HTT was made in only 8% of reported cases. More worrisome, however, is that the remaining 92% were misdiagnosed with false-positive results [6].
The classification of HTT as benign or malignant remains controversial. While it is generally regarded as benign, it is considered a borderline tumor with the potential for malignancy [7]. This diagnostic complexity has led to confusion in terminology, with HTT also being referred to by various other names, including hyalinizing trabecular adenoma, paraganglioma-like adenoma, hyalinizing trabecular neoplasm, and hyalinizing trabecular carcinoma [8]. This study aims to provide a comprehensive overview of HTT by retrospectively analyzing 11 cases, focusing on clinical presentations, diagnostic challenges, and treatment outcomes.
Methods
Study design
This study was a retrospective single-center case series. The patients were managed over five years, from January 2019 to November 2024. Data collection took place over one month, from November 15, 2024, to December 15, 2024. This study was approved by the Ethics Committee of Kscien organization (Approval No. 2025-33).
Participants
The study included all patients diagnosed with hyalinizing trabecular tumor. Diagnoses were confirmed through histopathological examination of resected thyroid tissue. Clinical and sociodemographic data were collected from patients, medical records, and healthcare providers.
Pre-intervention assessment
Assessments included vital signs monitoring, ultrasonography (U/S), thyroid function tests, serum calcium levels, vocal cord evaluation, viral screening (HBV, HCV, and HIV), and complete blood count.
Intervention
All patients underwent surgery under general anesthesia and were positioned supine with the neck extended and elevated using a roller placed beneath the shoulders. If visible, a 4 cm transverse collar incision was made in a natural skin crease of the lower neck for cosmetic purposes. Subplatysmal flaps were elevated superiorly and inferiorly to allow adequate thyroid gland exposure. A circular skin flap was then raised, with dissection carried laterally, medially, and toward the upper and lower cervical regions.
The midline between the strap muscles was divided, and the muscles were retracted laterally to expose the thyroid gland. Dissection commenced with ligation of the middle thyroid vein, followed by the superior and inferior pedicles. The superior and inferior thyroid vessels were ligated and divided close to the thyroid capsule to preserve the recurrent laryngeal nerves and avoid compromising the parathyroid gland vasculature. To minimize thermal injury, electrocautery use was limited; instead, multiple suture ligatures were applied to control oozing. Both sharp and blunt dissection techniques were employed to identify and preserve the recurrent laryngeal nerves, with all dissections maintained close to the thyroid capsule. The parathyroid glands were preserved in all cases, and surrounding adipose tissue was retained to maintain vascular integrity. In cases where devascularization was suspected, parathyroid autotransplantation into the sternocleidomastoid muscle was performed.
Of the 11 patients, 7 underwent total thyroidectomy. One of these patients underwent additional lateral and central neck dissection, with identification and preservation of the internal jugular vein, spinal accessory nerve, and phrenic nerve. The remaining 4 patients underwent lobectomy, performed using the same surgical technique but limited to the involved thyroid lobe.
After each procedure, hemostasis was confirmed. Closed suction drains (RediVac®) were placed in the patients. The strap muscles were reapproximated in the midline, and the skin was closed with absorbable subcuticular sutures and Steri-Strips. All patients tolerated the procedure without intraoperative complications.
Post-intervention Considerations
Postoperatively, all patients received intravenous paracetamol, and those who underwent total thyroidectomy were prescribed thyroid hormone replacement therapy (Levothyroxine) adjusted to their body weights. The diagnosis was confirmed through histopathological examination of the surgical specimens.
Results
Participants
The case series included 11 patients, of whom ten (90.9%) were female. Patient ages ranged from 32 to 85 years, with a mean age of 50.7±19.01 years. Seven patients (63.6%) had no significant past medical history, while three (27.3%) had hypertension, including one with concurrent type 2 diabetes and another with a history of renal stones. Surgical history was positive in six patients (55.5%). The most common presenting symptom was anterior neck swelling, observed in six patients (54.5%), followed by weight loss in three patients (27.3%). In one case (9.1%), the finding was incidental. Preoperative thyroid function assessment revealed hyperthyroidism in six patients (54.5%), and the remaining five (45.5%) were euthyroid (Table 1).
The left thyroid lobe was involved in five cases (45.5%), the right lobe in four cases (36.4%), and the isthmus in two cases (18.1%). The primary surgical approach was total thyroidectomy in seven cases (63.6%), including one patient who underwent concurrent neck dissection (Table 2). Tumor size ranged from 0.5 to 5.5 cm, with a mean size of 2.6 cm (Table 3). All the diagnoses were made post-operatively through histopathological examination (Figure 1).
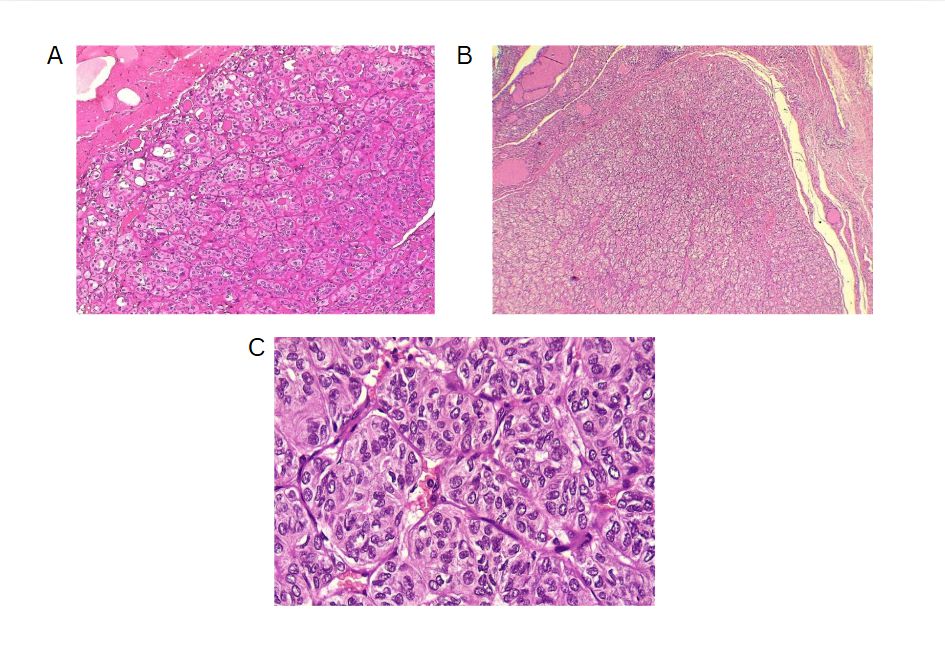
Outcomes and follow-up
The follow-up period ranged from one month to five years, with a mean duration of 2.3±1.27 years. During this period, no cases of recurrence were reported, and all patients achieved complete recovery. No significant complications were observed during or after the surgical procedures.
|
Case no. |
Age |
Sex |
Medical history |
Surgical history |
Drug history |
Chief complaint |
Duration |
Examination |
Thyroid function test |
|
1 |
39 |
F |
Unremarkable |
Unremarkable |
Unremarkable |
Incidental |
1 week |
Not palpable |
Hyperthyroid |
|
2 |
78 |
F |
Hypertension |
Cataract surgery & cholecystectomy |
Statin, Anticoagulant & Anti-Hypertension |
Shortness of breath |
8 years |
Hard |
Hyperthyroid |
|
3 |
74 |
M |
Unremarkable |
Kidney transplant |
Corticosteroids |
Weight-loss |
5 months |
Not palpable |
Hyperthyroid |
|
4 |
85 |
F |
Hypertension & renal stone |
Thyroid lobectomy & lithotripsy |
Anti-Hypertension |
Anterior neck swelling |
6 years |
Hard |
Euthyroid |
|
5 |
32 |
F |
Unremarkable |
Unremarkable |
Unremarkable |
Anterior neck swelling |
3 months |
Hard |
Euthyroid |
|
6 |
34 |
F |
Hodgkin’s lymphoma |
C-section & cervical cerclage |
Antibiotics |
Weight-loss |
8 months |
Not palpable |
Euthyroid |
|
7 |
38 |
F |
Unremarkable |
Cholecystectomy |
Unremarkable |
Anterior neck swelling |
1.5 years |
Hard |
Hyperthyroid |
|
8 |
42 |
F |
Unremarkable |
Unremarkable |
Unremarkable |
Anterior neck swelling |
1 year |
Hard |
Euthyroid |
|
9 |
51 |
F |
Hypertension & Type 2 Diabetes Mellitus |
Unremarkable |
Anti-Hypertension & Anti-diabetics |
Anterior neck swelling |
2 months |
Hard |
Hyperthyroid |
|
10 |
40 |
F |
Unremarkable |
Unremarkable |
Unremarkable |
Weight-loss |
6 months |
Not palpable |
Euthyroid |
|
11 |
45
|
F |
Unremarkable |
Unremarkable |
Unremarkable |
Anterior neck swelling |
1 year |
Hard |
Hyperthyroid |
|
M:Male, F:Female, S.Ca:Serum calcium, TG:Thyroglobulin, N/A:Not applicable |
|||||||||
|
Case no. |
S. Ca (mg/dL) |
US |
US consistency |
US echogenicity |
Side |
FNA Bethesda |
Vocal cord assessment |
Type of operation |
Post Op complications |
HPE |
Tumor Size (cm) |
Follow-up (years) |
|
1 |
N/A |
N/A |
N/A |
N/A |
Left lobe |
V |
Normal |
Lobectomy |
None |
HTT |
1.4 |
5 |
|
2 |
9.6 |
TR3 |
Mixed |
Heterogenous |
Left lobe |
IV |
Normal |
Total thyroidectomy |
None |
HTT |
3.8 |
2 |
|
3 |
7.9 |
GD |
No nodule |
No nodule |
Isthmus |
NP |
Normal |
Total thyroidectomy |
None |
HTT |
1 |
2 |
|
4 |
9.8 |
GD |
No nodule |
No nodule |
Right lobe |
NP |
Normal |
Total thyroidectomy |
None |
HTT |
6 |
0.08 |
|
5 |
9.2 |
TR4 |
Solid |
Hyperechoic |
Left lobe |
V |
Normal |
Total thyroidectomy |
None |
HTT |
4.5 |
3 |
|
6 |
9.5 |
TR4 |
Solid |
Hyperechoic |
Left lobe |
V |
Normal |
Total thyroidectomy |
None |
HTT |
0.5 |
3 |
|
7 |
9.1 |
TR3 |
Solid |
Heterogenous |
Left lobe |
NP |
Normal |
Lobectomy |
None |
HTT |
1.4 |
1 |
|
8 |
9.5 |
TR4 |
Solid |
Hypoechoic |
Right lobe |
IV |
Normal |
Lobectomy |
None |
HTT |
3 |
2 |
|
9 |
9 |
TR3 |
N/A |
N/A |
Isthmus |
NP |
Normal |
Total thyroidectomy |
None |
HTT |
0.8 |
2 |
|
10 |
9.2 |
TR3 |
N/A |
N/A |
Right lobe |
III |
Normal |
Total thyroidectomy |
None |
HTT |
0.7 |
3 |
|
11 |
8.9 |
TR3 |
N/A |
N/A |
Right lobe |
NP |
Normal |
Lobectomy |
None |
HTT |
5.5
|
3
|
|
N/A: Not Applicable, S.Ca: Serum Calcium, US: Ultrasound, FNA: Fine Needle Aspiration, OP: Operation, HPE: Histopathology, TR: TI-RADS, GD: Graves' Disease, NP: Non-Productive, HTT: Hyalinizing trabecular tumor |
||||||||||||
|
Variables |
Frequency |
|
Sex |
|
|
Male |
1 (9.1%) |
|
Female |
10 (90.9) |
|
Age groups (years) |
|
|
30-39 |
4 (36.3%) |
|
40-49 |
3 (27.3%) |
|
50-59 |
1 (9.1%) |
|
>60 |
3 (27.3%) |
|
Mean ± SD |
50.7 ± 19.0 |
|
Medical history |
|
|
Unremarkable |
7 (63.6%) |
|
Hypertension |
1 (9.1%) |
|
Hypertension & renal stones |
1 (9.1%) |
|
Hodgkin’s lymphoma |
1 (9.1%) |
|
Hypertension & Type 2 Diabetes Mellitus |
1 (9.1%) |
|
Surgical history |
|
|
Unremarkable |
6 (54.5%) |
|
Cholecystectomy |
1 (9.1%) |
|
C-section & cervical cerclage |
1 (9.1%) |
|
Cholecystectomy & cataract surgery |
1 (9.1%) |
|
Kidney transplant |
1 (9.1%) |
|
Thyroid lobectomy & lithotripsy |
1 (9.1%) |
|
Drug history |
|
|
Negative |
6 (54.5%) |
|
Corticosteroids |
1 (9.1%) |
|
Antibiotics |
1 (9.1%) |
|
Antihypertensive |
1 (9.1%) |
|
Antihypertensive & Antidiabetic |
1 (9.1%) |
|
Chief complaint |
|
|
Anterior neck swelling |
6 (54.5%) |
|
Shortness of breath |
1 (9.1%) |
|
Weight loss |
3 (27.3%) |
|
Incidental |
1 (9.1%) |
|
Thyroid examination |
|
|
Not palpable |
7 (63.6%) |
|
Hard |
4 (36.4%) |
|
Thyroid function |
|
|
Hyperthyroid |
6 (54.5%) |
|
Euthyroid |
5 (45.5%) |
|
Affected side |
|
|
Right lobe |
4 (36.3%) |
|
Left lobe |
5 (45.5%) |
|
Isthmus |
2 (18.2%) |
|
Operation |
|
|
Total thyroidectomy |
7 (63.6%) |
|
Lobectomy |
4 (36.4%) |
|
Tumor size (cm) |
|
|
Mean ± SD |
2.6 ± 1.95 |
|
Follow-up (years) |
|
|
0.0 - 1.0 |
2 (18.18%) |
|
1.1 - 2.0 |
4 (36.36%) |
|
2.1 - 3.0 |
4 (36.36%) |
|
3.1 - 4.0 |
0 (0.0%) |
|
4.1 - 5.0 |
1 (9.09 %) |
|
Mean ± SD |
2.3 ± 1.27 |
Discussion
The diagnosis of HTT is challenging due to its resemblance to other thyroid neoplasms. While most cases are asymptomatic, Rossi et al. stated that symptom presentation may depend on tumor size and location [5]. Among the 11 cases, only one was asymptomatic, while the remaining ten exhibited clinical symptoms, including anterior neck swelling, weight loss, and shortness of breath. However, in the six reviewed cases, three were asymptomatic, two exhibited neck swelling, and one experienced both dyspnea and dysphagia (Table 4) [2,3,7-10].
|
Author/year |
Age |
Sex |
Medical history |
Drug history |
Surgical history |
Chief complaint |
Examination |
Duration (years) |
Side
|
Size (cm)* |
Distant metastasis |
TI-RADS |
FNA findings |
Therapeutic approach |
IHC findings |
Diagnosis |
Outcome |
Follow-up (years) |
|
Zhang et al./2025 [7] |
31 |
F |
Unremarkable |
Unremarkable |
Unremarkable |
Incidental |
Palpable mass |
1.5 |
R |
2.2 |
No |
TR3 |
N/A |
Lobectomy |
TG +ve, CK19 +ve, TTF-1 +ve & Ki-67 5% |
HTT |
Resolved |
0.6 |
|
Hayashi et al./2025 [2] |
93 |
F |
Diabetes mellitus, hyperlipidemia, hypertension & myocardial infarction |
N/A |
N/A |
Loss of appetite, dyspnea & dysphagia |
Enlarged, firm, non-tender, without palpable nodules |
>12 |
B |
>10 |
No |
N/A |
N/A |
Conservative management & rehabilitation |
N/A |
HTT |
Improved |
N/A |
|
Alsogair et al./2023 [3] |
60 |
M |
Unremarkable |
Unremarkable |
Hemorrhoidectomy |
Mass on the neck |
Palpable, firm & non-tender |
N/A |
R |
3.8 |
No |
N/A |
60%-75% likelihood of malignancy |
Total thyroidectomy followed by thyroxine |
TG +ve & Ki-67 +ve |
HTT |
Resolved |
0.06 |
|
Katano et al./ 2021 [10] |
54 |
F |
Panic disorder & chronic thyroiditis |
Unremarkable |
Unremarkable |
Left cervical mass |
Growing, painless & elastic |
N/A |
L |
4.5 |
No |
N/A |
Chronic thyroiditis with possible malignancy |
Lobectomy |
Ki-67 +ve for cytoplasm & ColIV +ve |
HTT |
Resolved |
1.5 |
|
Rhee et al./2018 [9] |
63 |
F |
Breast cancer |
N/A |
N/A |
Incidental |
N/A |
N/A |
L |
0.6 |
No |
N/A |
Features of PTC |
Lobectomy |
Ki-67 +ve, CD56 +ve & Galectin-3 +ve |
HTT |
Resolved |
N/A |
|
Jones et al./2017 [8] |
70 |
F |
N/A |
N/A |
N/A |
Incidental |
N/A |
N/A |
R |
1.94 |
N/A |
N/A |
60-75% likelihood of malignancy |
Total thyroidectomy |
TG +ve, vimentin +ve & CK19 +ve |
HTT |
Resolved |
0.08 |
|
M: Male, F: Female, N/A: Not applicable, FNA: Fine needle aspiration, IHC: Immunohistochemistry, HTT: Hyalinizing trabecular tumor, L: Left, R: Right, B: Both |
||||||||||||||||||
The diagnostic evaluation of most thyroid nodules typically begins with U/S, followed by fine needle aspiration (FNA). The U/S findings suggestive of HTT are well-defined, solitary, oval or round, solid hypoechoic nodules, usually without microcalcifications and displaying peri or intra-nodular vascularity. However, these features are not specific to HTT and may also occur in other thyroid lesions [7]. Recognizing the variability in U/S findings is crucial, as some studies reported an absence of malignant features. In contrast, Choi et al. found that 29% of HTT cases displayed malignant features on U/S [5,11]. In the present study, five cases were classified as mildly suspicious for malignancy, and three were considered moderately suspicious for malignancy according to the thyroid imaging reporting and data system (TI-RADS). Among the reviewed cases, malignancy was also suspected in four patients based on the U/S findings [2,3,7-10].
The primary diagnostic tool for thyroid nodules is FNA, which often leads to the misclassification of HTT as PTC or medullary thyroid carcinoma [9]. Ito et al. suggested that this diagnostic confusion arises from shared cytological features, including intranuclear cytoplasmic inclusions and nuclear grooves, which represent hallmark characteristics of PTC [4]. The cytological appearance of HTT on liquid-based preparations reveals cohesive aggregates or syncytial fragments of tumor cells surrounding hyaline material. Although tumor cells in HTT show enlarged nuclei with hyperchromasia and occasional intranuclear pseudo-inclusions similar to papillary carcinoma, HTT cells typically display dispersed fine chromatin rather than the pale and clear chromatin pattern observed in PTC [9].
Additionally, HTT cells demonstrate less frequent nuclear membrane irregularity and exhibit a more stratified trabecular arrangement compared to papillary carcinoma. These subtle distinctions prove crucial for accurate cytological interpretation, though they remain challenging to discern consistently in clinical practice [9]. Dell’Aquila et al. reported that up to 75% of HTTs are classified within Bethesda categories IV to VI [12]. Among the cases included, two were diagnosed as Bethesda category IV, while three were classified as category V, emphasizing their frequent misinterpretation by cytopathologists. Equivocal cytomorphologic diagnoses, such as atypia of undetermined significance or follicular lesion of undetermined significance, require repeat FNA, as the malignancy risk for nodules in these categories ranges from 1% to 15% [8].
On gross examination, HTT typically presents as a solid, well-circumscribed mass, or less commonly, as an encapsulated tumor, with colors ranging from yellow to tan, opposite to PTC, which is usually white and does not have a capsule. HTT generally lacks invasion into the capsule, vasculature, or thyroid parenchyma [5,7]. However, Gowrishankar reported a case in which invasion and malignant behavior were observed in HTT [13].
Immunohistochemistry can aid in diagnosing HTT, although some biomarkers used may lack significant specificity. HBME-1 and galectin-3 are well-established markers for malignant thyroid lesions, particularly PTC and its variants. However, their expression in HTT
remains a subject of debate. In their series, Dell’Aquila et al. found that the majority of HTT cases exhibited a distinct immune profile, with negative immunoreactivity observed in 16 out of 18 (89%) lesions. This finding further supports the classification of HTT as a benign tumor [12].
Recent genetic studies have demonstrated that GLIS rearrangements, particularly the PAX8-GLIS3 gene fusion, are critical for diagnosing HTT. Research indicates this fusion was present in 93% of HTT cases (13 out of 14), with the remaining 7% involving a PAX8-GLIS1 rearrangement. These findings highlight the diagnostic utility of detecting GLIS-related fusions to distinguish HTT from morphologically similar thyroid neoplasms [4].
In 2012, Smith et al. suggested that HTT could potentially acquire mutations leading to RET/PTC expression and undergo malignant transformation into PTC [14]. Given the uncertainty regarding the malignant potential of HTT, treatment approaches typically involve complete resection, near-total thyroidectomy, or lobectomy [8]. However, evidence suggests that up to three-quarters of patients may be subjected to overtreatment, opting for total or subtotal thyroidectomy rather than the less invasive lobectomy. In contrast, some experts argue for a more conservative management strategy, advocating for close monitoring or lobectomy as a first-line approach, rather than resorting to total thyroidectomy immediately [8]. Among the cases included in the current series, seven patients underwent total thyroidectomy, accounting for 63.6% of the surgeries performed. Utilizing total thyroidectomy as the surgical method mainly resulted from uncertainty in diagnosis, as imaging and other pre-operative examinations don’t usually provide a solid diagnosis.
Conclusion
In conclusion, HTT is a rare tumor that is challenging to diagnose accurately. Both total thyroidectomy and lobectomy may result in good outcomes.
Declarations
Conflicts of interest: The authors have no conflicts of interest to disclose.
Ethical approval: Ethical approval for this study was obtained from the Ethics Committee of the Kscien Organization (Approval No. 2025-33)
Consent for participation: Not applicable.
Consent for publication: Written informed consent for publication was obtained from all patients.
Funding: The present study received no financial support.
Acknowledgments: None to be declared.
Authors' contributions: AMS, ROM, FHK, and MMA: Major contributors to the conception of the study, as well as the literature search for related studies, and manuscript writing. HOB, SHH, MBA, IJH, ISS, and DQH: Literature review, design of the study, critical revision of the manuscript, and processing of the tables. KKM, MNH, AAQ, HAA, and HKM: Literature review and processing of the figure. All authors have read and approved the final version of the manuscript.
Use of AI: ChatGPT-3.5 was used to assist with language refinement and improve the overall clarity of the manuscript. All content was thoroughly reviewed and approved by the authors, who bear full responsibility for the final version.
Data availability statement: Not applicable.
References
- Abdullah AM, Qaradakhy AJ, Ali RM, Ali RM, Mahmood YM, Omar SS, et al. Thyroid Collision Tumors: A Systematic Review. Barw Medical Journal. 2024;2(2):44-56. doi:10.58742/bmj.v2i2.94.
- Hayashi K, Nakaya Y, Yoshida Y, Hayashi M, Izumi R, Suzuki A et al. Dysphagia and Dyspnea Caused by a Giant Hyalinizing Trabecular Tumor: A Case Report. Cureus. 2025;17(2). doi:10.7759/cureus.79044
- Alsogair O, Alalawi AA, Alzahim AF, Saleem MA, Aljohani FM, Alahmadi LS et al. Hyalinizing trabecular tumor of the thyroid gland: A case report and literature review. Cureus. 2023;15(4). doi:10.7759/cureus.37845
- Ito Y, Hirokawa M, Kousaka K, Ito M, Kihara M, Miya A et al. Diagnosis and management of hyalinizing trabecular tumor of the thyroid: a single-institution experience. Endocrine Journal. 2021;68(12):1403-9. doi:10.1507/endocrj.EJ21-0143
- Rossi ED, Papotti M, Faquin W, Larocca LM, Pantanowitz L. The diagnosis of hyalinizing trabecular tumor: a difficult and controversial thyroid entity. Head and Neck Pathology. 2020;14(3):778-84. doi:10.1007/s12105-019-01083-5
- Saglietti C, Piana S, La Rosa S, Bongiovanni M. Hyalinizing trabecular tumour of the thyroid: fine-needle aspiration cytological diagnosis and correlation with histology. Journal of clinical pathology. 2017;70(8):641-7. doi:10.1136/jclinpath-2017-204360
- Zhang L, Ma Q, Shen Z, Guo L. Hyalinizing trabecular tumor of the thyroid: A case report. Oncology Letters. 2025;29(3). doi:10.3892/ol.2025.14865
- Jones DJ, Kieliszak CR, Patel SS, Selinsky CR. Hyalinizing trabecular tumor of the thyroid gland and its significant diagnostic issue. Thyroid research. 2017;10:1-5. doi:10.1186/s13044-017-0042-5
- Rhee YY, Jung HK, Kim SH, Kim SH. Hyalinizing trabecular tumor of the thyroid gland, a diagnostic challenge in fine-needle aspiration cytology: Case report. Journal of Pathology and Translational Medicine. 2018;52(4):252-6. doi:10.4132/jptm.2018.04.28
- Katano H, Hasegawa H, Matsuzaki H, Oshima T, Tang X. Thyroid hyalinizing trabecular adenoma with a high thyroglobulin level: a case report. Journal of Surgical Case Reports. 2021;2021(7):rjab324. doi:10.1093/jscr/rjab324.
- Choi WJ, Baek JH, Ha EJ, Choi YJ, Hong MJ, Song DE et al. The ultrasonography features of hyalinizing trabecular tumor of the thyroid gland and the role of fine needle aspiration cytology and core needle biopsy in its diagnosis. Acta Radiologica. 2015;56(9):1113-8. doi:10.1177/0284185114549225.
- Dell’Aquila M, Gravina C, Cocomazzi A, Capodimonti S, Musarra T, Sfregola S et al. A large series of hyalinizing trabecular tumors: Cytomorphology and ancillary techniques on fine needle aspiration. Cancer cytopathology. 2019;127(6):390-8. doi:10.1002/cncy.22139
- Gowrishankar S, Pai SA, Carney JA. Hyalinizing trabecular carcinoma of the thyroid gland. Histopathology. 2008;52(4):529-31. doi:10.1111/j.1365-2559.2008.02945.x.
- Smith NR, Bullock MJ, Hart RD, Trites JR, Taylor SM. Hyalinizing trabecular tumour: review and new insights into the molecular biology. J Otolaryngol Head Neck Surg 2012;41(1):30-34. doi:10.1111/j.1365-2559.2008.02945.x.
Copyright (c) 2025 The Author(s)

This work is licensed under a Creative Commons Attribution 4.0 International License.