Vol 1, No 2 (2025): Current Issue (Volume 1, Issue 2), 2025
Original Article
Preoperative Thyroglobulin and Thyroid Pathologies: A Single Center Experience
Shaho F. Ahmed, Rebaz M. Ali, Rawa M. Ali, Ari M. Abdullah, Abdulwahid M. Salih, Hiwa O....
Abstract
Introduction: Thyroid nodules are frequently found in the general population, though malignancy is confirmed in only a minority of cases. Distinguishing between benign and malignant nodules before surgery is vital for appropriate clinical management. The utility of preoperative serum thyroglobulin (Tg) levels as a diagnostic marker in thyroid carcinomas remains controversial. This study aimed to provide a descriptive overview of patients with markedly elevated preoperative Tg levels (>500 ng/mL) and their corresponding histopathological outcomes.
Methods: A retrospective, single-center study was conducted at Smart Health Tower, which included patients who underwent surgical interventions for thyroid disorders between 2019 and 2025, with preoperative serum Tg levels exceeding 500 ng/mL. Patients were excluded if they had incomplete medical records. Patient demographics, clinical features, preoperative findings, surgical details, and final histopathology were retrieved from electronic medical records.
Results: A total of 260 patients were included, predominantly female (73.08%), with a median age of 49 years (QR: 38–61). Neck swelling was the most common symptom (63.08%). Ultrasonography showed follicular nodular disease in 60.0%. Fine-needle aspiration cytology revealed Bethesda II in 20.0%, IV in 13.85%, and VI in 11.15% of cases. Total thyroidectomy was performed in 74.62% of cases. Histopathology showed benign lesions in 70.77% and malignant lesions in 29.23% of the cases.
Conclusion: Preoperative Tg levels may be elevated in both benign and malignant thyroid disorders; however, Tg may not possess adequate diagnostic precision to be used as a sole indicator of malignancy.
Introduction
Thyroid nodules are highly prevalent in the general population, with ultrasonography identifying them in up to 60% of individuals. Despite their frequency, approximately 5% are malignant [1, 2].
Primary thyroid carcinoma is the most common endocrine malignancy and is broadly classified into follicular epithelial-derived and non-follicular epithelial-derived tumors. Tumors of follicular epithelial origin include differentiated thyroid carcinoma (DTC), poorly differentiated carcinoma, and undifferentiated (anaplastic) carcinoma. DTC, which encompasses papillary thyroid carcinoma (PTC), follicular thyroid carcinoma, and oncocytic (previously Hürthle), accounts for approximately 95% of all thyroid malignancies [3, 4].
Accurate preoperative identification of thyroid nodules as benign or malignant is essential for guiding appropriate treatment decisions [3]. Initial evaluation commonly involves thyroid ultrasound, which plays a vital role in differentiating benign from malignant nodules. Complementary diagnostic techniques, such as contrast-enhanced ultrasound and ultrasonic elastography, provide additional insight. Among these, ultrasound-guided fine needle aspiration (FNA) is considered the most reliable method for diagnosing DTC prior to surgery. However, the accuracy of both ultrasound and FNA can be affected by factors including nodule size, sonographic features, and the clinician’s experience [5].
Reliable serum biomarkers are also essential for diagnosing and managing DTC. Thyroglobulin (Tg), a large glycoprotein composed of 2750 amino acids with a molecular weight of approximately 330 kilodaltons, is synthesized and secreted by thyroid follicular epithelial cells. It is primarily localized within thyroid follicular structures, with only minimal quantities circulating in the bloodstream under normal physiological conditions [6]. In patients with DTC who have undergone total thyroidectomy, particularly those who receive radioactive iodine (I-131) therapy, serum Tg functions as a valuable biomarker for assessing residual thyroid tissue, disease recurrence, and distant metastases, as its presence in the circulation postoperatively is typically indicative of persistent or recurrent disease [3].
The clinical significance of preoperative Tg levels as a diagnostic and prognostic tool in thyroid carcinomas remains a subject of ongoing debate [3]. This study aimed to provide a descriptive overview of patients with markedly elevated preoperative Tg levels (>500 ng/mL, reference range 3–40 ng/mL) and their corresponding histopathological findings following thyroid surgery. All references were evaluated for eligibility using reputable predatory journal lists to ensure scholarly integrity [7].
Methods
Study design
This retrospective, single-center study was conducted at the Smart Health Tower (Sulaymaniyah, Iraq) and included patients who underwent surgical interventions for thyroid disorders between 2019 and 2025. The study received approval from the Kscien Organization’s Ethics Committee under registration number 40 in 2025.
Inclusion Criteria
The study included only patients who had elevated preoperative serum Tg levels exceeding 500 ng/mL. The threshold of 500 ng/mL was selected because it represents a markedly abnormal elevation well beyond the ranges commonly reported in both benign and malignant thyroid disease in previous studies [8–14]. In addition, this value corresponded to the upper reporting limit of our institutional laboratory assay, which does not quantify Tg concentrations above 500 ng/mL.
Exclusion Criteria
Patients were excluded if they had incomplete medical records.
Data Collection
Demographic and clinical data were retrieved from electronic medical records. The variables collected included age, sex, occupation, relevant medical history, presenting symptoms, results of preoperative imaging and laboratory investigations, Bethesda category, type of surgery performed, and final histopathological diagnosis.
Statistical Analysis
All collected data were entered into Microsoft Excel and subsequently analyzed using IBM SPSS (Statistical Package for the Social Sciences), version 27.0. Continuous variables were presented as means with standard deviations or medians with quartile ranges, as appropriate, while categorical variables were summarized using frequencies and percentages.
Results
Patient Demographics
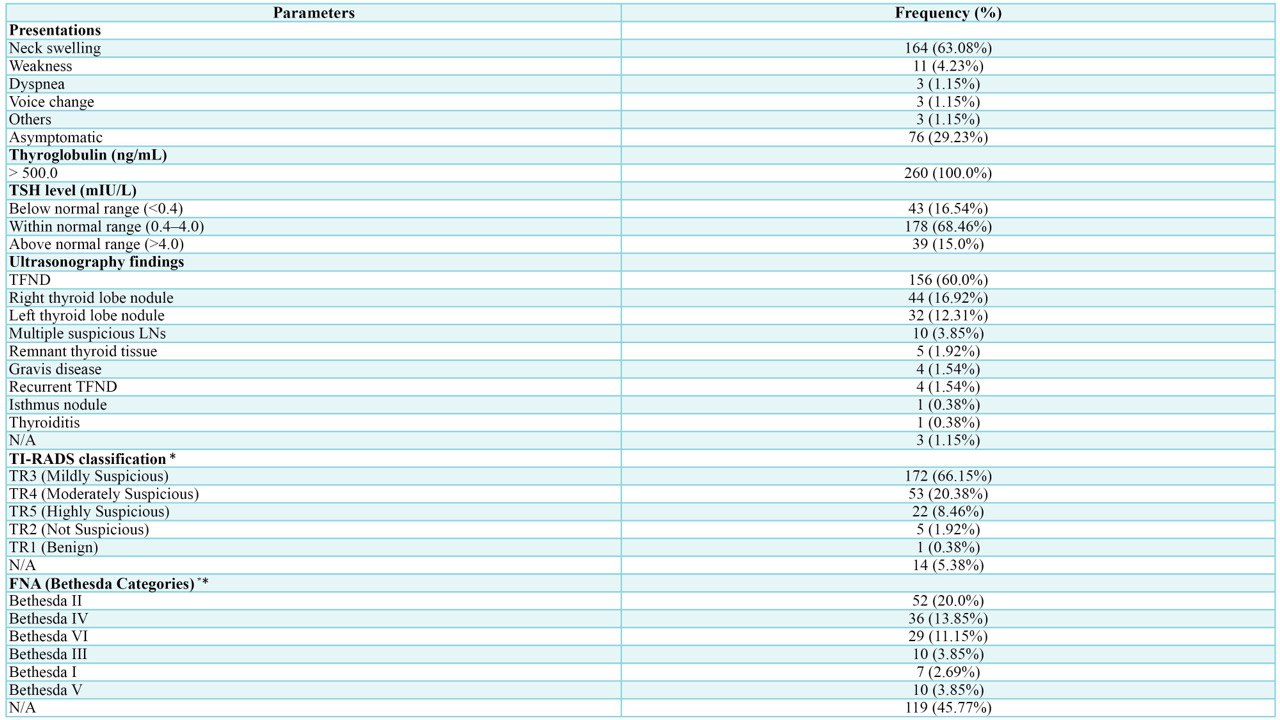
A total of 260 patients were included in this study. The majority were female (n = 190, 73.08%), while the remaining 70 patients (26.92%) were male. The median age was 49 years (QR: 38–61). The highest frequency of patients was observed in the 40–49 age group (n = 61, 23.46%), followed by the 50–59 age group (n = 53, 20.38%). In terms of smoking, 216 patients (83.08%) were non-smokers. The most common occupation among the patients was homemaker (n = 150, 57.69%) (Table 1).
|
Parameters |
Frequency (%) |
|
Gender Female Male |
190 (73.08%) 70 (26.92%) |
|
Age (year) < 20 20 – 29 30 – 39 40 – 49 50 – 59 60 – 69 70 – 79 > 80 Median (QR) Mean ± SD |
5 (1.92%) 18 (6.92%) 50 (19.23%) 61 (23.46%) 53 (20.38%) 48 (18.46%) 24 (9.23%) 1 (0.38%) 49 (38 – 61) 49.14 ± 14.76 |
|
Smoking status Active smoker Passive smoker Former smoker Non-smoker |
30 (11.54%) 13 (5.00%) 1 (0.38%) 216 (83.08%) |
|
Occupation Homemaker Teacher Unemployed Retired Police officer Student Butcher Engineer Farmer Healthcare personnel Others |
150 (57.69%) 15 (5.77%) 11 (4.23%) 11 (4.23%) 6 (2.31%) 5 (1.92%) 1 (0.38%) 1 (0.38%) 1 (0.38%) 1 (0.38%) 58 (22.31%) |
|
Medical history* Unremarkable Hypertension Diabetes mellitus Heart disease Asthma Deaf Stroke |
243 (93.46%) 13 (5.0%) 7 (2.69%) 4 (1.54%) 1 (0.38%) 1 (0.38%) 1 (0.38%) |
|
Past surgical history Unremarkable Total thyroidectomy Right thyroid lobectomy CABG |
238 (91.54%) 19 (7.30%) 2 (0.77%) 1 (0.38%) |
|
*: Some patients had multiple medical conditions, CABG: Coronary artery bypass graft, IQR: Interquartile range, SD: Standard deviation |
|
Medical and Surgical History
Two hundred forty-three patients (93.46%) had no past medical conditions. Hypertension was present in 13 patients (5.0%). Most patients (n = 238, 91.54%) had no prior surgical history. Nineteen patients (7.30%) had previously undergone total thyroidectomy, and 2 (0.77%) had undergone right thyroid lobectomy (Table 1).
Presenting Symptoms and Laboratory Findings
The most common presenting symptom was neck swelling, reported in 164 patients (63.08%), while 76 patients (29.23%) were asymptomatic. All patients had preoperative elevated serum Tg levels exceeding 500 ng/mL. Thyroid-stimulating hormone (TSH) levels were within the normal range in 178 patients (68.46%) (Table 2).
|
Parameters |
Frequency (%) |
|
Presentations Neck swelling Weakness Dyspnea Voice change Others Asymptomatic |
164 (63.08%) 11 (4.23%) 3 (1.15%) 3 (1.15%) 3 (1.15%) 76 (29.23%) |
|
Thyroglobulin (ng/mL) > 500.0 |
260 (100.0%) |
|
TSH level (mIU/L) Below normal range (<0.4) Within normal range (0.4–4.0) Above normal range (>4.0) |
43 (16.54%) 178 (68.46%) 39 (15.0%) |
|
Ultrasonography findings TFND Right thyroid lobe nodule Left thyroid lobe nodule Multiple suspicious LNs Remnant thyroid tissue Gravis disease Recurrent TFND Isthmus nodule Thyroiditis N/A |
156 (60.0%) 44 (16.92%) 32 (12.31%) 10 (3.85%) 5 (1.92%) 4 (1.54%) 4 (1.54%) 1 (0.38%) 1 (0.38%) 3 (1.15%) |
|
TI-RADS classification * TR3 (Mildly Suspicious) TR4 (Moderately Suspicious) TR5 (Highly Suspicious) TR2 (Not Suspicious) TR1 (Benign) N/A |
172 (66.15%) 53 (20.38%) 22 (8.46%) 5 (1.92%) 1 (0.38%) 14 (5.38%) |
|
FNA (Bethesda Categories) ** Bethesda II Bethesda IV Bethesda VI Bethesda III Bethesda I Bethesda V N/A |
52 (20.0%) 36 (13.85%) 29 (11.15%) 10 (3.85%) 7 (2.69%) 10 (3.85%) 119 (45.77%) |
|
*: TI-RADS classifications were assigned to multiple nodules in certain patients, **: Some patients underwent FNA more than once, TFND: Thyroid follicular nodular disease, N/A: Not available, TI-RADS: Thyroid imaging reporting and data system, FNA: Fine needle aspiration, LN: Lymph node |
|
Ultrasonographic and Fine-Needle Aspiration Findings
Ultrasonographic evaluation revealed thyroid follicular nodular disease (TFND) in 156 patients (60.0%), right thyroid lobe nodules in 44 (16.92%), and left lobe nodules in 32 (12.31%). According to the TI-RADS classification, most nodules were TR3 (n = 172, 66.15%), followed by TR4 (n = 53, 20.38%) and TR5 (n = 22, 8.46%). FNA findings based on the Bethesda system were available for 141 patients (54.23%). The most frequent categories were Bethesda II (n = 52, 20.0%), Bethesda IV (n = 36, 13.85%), and Bethesda VI (n = 29, 11.15%) (Table 2).
Surgical Intervention
Surgical intervention consisted predominantly of total thyroidectomy (n = 194, 74.62%). Central neck dissection was performed in 18 patients (6.92%), while lateral neck dissection was carried out in 11 patients (4.23%) (Table 3).
|
Parameters |
Frequency (%) |
|
Type of operation Total Thyroidectomy Thyroid lobectomy Thyroid nodulectomy Completion lobectomy Isthmusectomy Revision of thyroid remnant |
194 (74.62%) 46 (17.69%) 15 (5.77%) 2 (0.77%) 2 (0.77%) 1 (0.38%) |
|
Central neck dissection Bilateral central neck dissection Right central neck dissection Left central neck dissection Not performed |
8 (3.08%) 6 (2.31%) 4 (1.54%) 242 (93.07%) |
|
Lateral neck dissection Left lateral neck dissection level Right lateral neck dissection level Bilateral lateral neck dissection level Not performed |
5 (1.92%) 5 (1.92%) 1 (0.38%) 249 (95.77%) |
|
Histopathological diagnosis TFND PTC Conventional variant of PTC Conventional variant of PTMC Conventional and follicular variants of PTC Follicular variant of PTC Tall cell variant of PTC Conventional and trabecular variants of PTC Infiltrative follicular subtype of PTC Hyperplastic nodule Adenomatoid nodule Minimally invasive follicular carcinoma NIFTP Follicular adenoma Graves' Disease Hashimoto's thyroiditis Oncocytic adenoma Oncocytic cell carcinoma Differentiated high-grade thyroid carcinoma Hyalinizing trabecular adenoma Benign cystic thyroid colloid nodule Collision tumors (PTC and high-grade FTC) Collision tumors (PTC and Oncocytic cell carcinoma) Follicular thyroid carcinoma FT-UMP Insular thyroid carcinoma Infarcted oncocytic cell lesion Invasive EFVPTC MTC |
115 (44.23%) 56 (21.54%) 38 (67.86%) 9 (16.07%) 3 (5.35%) 2 (3.57%) 2 (3.57%) 1 (1.79%) 1 (1.79%) 28 (10.77%) 10 (3.85%) 9 (3.46%) 7 (2.69%) 6 (2.31%) 6 (2.31%) 4 (1.54%) 3 (1.15%) 3 (1.15%) 2 (0.77%) 2 (0.77%) 1 (0.38%) 1 (0.38%) 1 (0.38%) 1 (0.38%) 1 (0.38%) 1 (0.38%) 1 (0.38%) 1 (0.38%) 1 (0.38%) |
|
Malignancy Status Benign Malignant |
184 (70.77%) 76 (29.23%) |
|
EFVPTC: Encapsulated follicular variant of papillary thyroid carcinoma, FT-UMP: Follicular tumor of uncertain malignant potential, MTC: Medullary thyroid carcinoma, NIFTP: Non-invasive follicular thyroid neoplasm with papillary-like nuclear features, PTMC: Papillary thyroid microcarcinoma, PTC: Papillary thyroid carcinoma |
|
Histopathological Findings
Histopathological analysis showed that the most common diagnosis was TFND (n = 115, 44.23%), followed by PTC in 56 patients (21.54%). Among PTC cases, the conventional variant was the most frequent (n = 38, 67.86%). Hyperplastic nodules were reported in 28 patients (10.77%), and adenomatoid nodules in 10 (3.85%). In total, 184 patients (70.77%) were diagnosed with benign pathology, whereas 76 patients (29.23%) were confirmed to have malignant disease (Table 3).
Discussion
The production of Tg is influenced by various internal physiological factors, such as hyperthyroidism due to Graves' disease, TSH levels, and the size of the thyroid gland and nodules [15]. Tg binds covalently to iodine and is stored in the follicular lumen until stimulated by TSH, which triggers its reabsorption into thyroid cells and its enzymatic breakdown into the active thyroid hormones, T3 and T4. These hormones are then released into the bloodstream and can be measured using peripheral blood assays. While T3 and T4 are the primary hormones secreted, small amounts of Tg also enter the circulation and can be detected at low levels in healthy individuals with normal thyroid function. However, in individuals with DTCs originating from follicular cells, Tg appears to be actively secreted [15].
Since Tg is produced solely by thyroid follicular cells, measuring its levels in the blood following a total thyroidectomy for thyroid cancer has become a standard approach for identifying persistent or recurrent disease. A serum Tg concentration of 1.0 ng/mL or higher is considered a highly reliable indicator of tumor recurrence [15].
Ultrasonography and FNA are primary diagnostic tools used to distinguish benign from malignant thyroid nodules before surgery. While FNA is commonly employed for this purpose, both methods have notable limitations, particularly in accurately differentiating benign from malignant follicular tumors [3]. As a result, approximately 20% to 30% of thyroid nodules still require surgical intervention to obtain a definitive pathological diagnosis following FNA [3]. The current study's findings support this observation, as FNA results were available for only about half of the patients (54.23%), and a substantial proportion of nodules, especially those classified as Bethesda category IV or VI, still required surgical confirmation.
Preoperative serum Tg levels are advantageous due to their convenience, rapid availability, and consistent reproducibility. However, their effectiveness in differentiating benign from malignant thyroid nodules before surgery has not been conclusively established. Despite this, many studies have investigated the potential of preoperative Tg levels for this purpose in diverse patient populations [3].
Several studies have demonstrated a significant association between elevated preoperative Tg levels and the presence of thyroid malignancy. Melik et al. (2022) prospectively measured Tg levels in 203 patients undergoing total thyroidectomy and found that patients with PTC had significantly higher Tg levels (105.05 ng/mL) compared to those with benign disease (76.80 ng/mL). A receiver operating characteristic analysis identified a cutoff value of 102 ng/mL, with the difference reaching statistical significance (P < 0.05) [8]. Similarly, Petric et al. (2012) observed higher preoperative Tg levels in PTC patients relative to those with benign nodules [9]. Jin et al. (2022) further supported this association in a cohort study that included 500 PTC patients, 376 with benign nodules, and 125 healthy controls. After excluding patients positive for Tg antibodies, they reported significantly higher Tg levels in PTC patients (42.87 ng/mL) compared to those with benign nodules (33.13 ng/mL) and healthy individuals (14.90 ng/mL), though the difference between the benign and healthy groups was not significant [10]. In the present study, PTC was the most prevalent subtype among malignant cases with thyroglobulin levels exceeding 500 ng/mL, accounting for 73.68% of such cases. The conventional variant was the most common among these, representing 67.86% of the PTC cases.
In a broader evaluation, Lu et al. (2024) conducted a systematic review and meta-analysis. They found that, among patients with indeterminate cytology and negative Tg antibodies, those with malignant nodules had significantly higher serum Tg levels than those with benign nodules (OR = 2.59, 95% CI: 1.59–4.21, P < 0.001) [3].
Conversely, several studies have reported the opposite trend, with lower preoperative Tg levels observed in patients with DTC compared to those with benign thyroid nodules. Zhang et al. (2022) analyzed 1,519 DTC cases and 571 benign cases, finding significantly lower Tg levels in the DTC group (15.7 ng/mL vs. 52.1 ng/mL, P < 0.001) [11]. Aydogu et al. (2023) similarly found that patients with benign nodules had higher Tg levels than those with DTC (85.0 ng/mL vs. 27.15 ng/mL, P < 0.001) [12]. Kang et al. (2022) reported consistent findings, noting lower average Tg levels in DTC patients (238 ± 77 ng/mL) compared to those with benign nodules (532 ± 97 ng/mL, P = 0.02) [13]. Patel et al. (2019) echoed this trend, with a median Tg level of 167.5 ng/mL in benign cases compared to 30.8 ng/mL in malignant ones (P < 0.001) [14].
The present study found that only 29.23% of the cases were diagnosed with thyroid malignancy, primarily PTC, while the majority (70.77%) had benign pathology, predominantly TFND. These results align with those of Zhang et al. [11], Aydogdu et al. [12], Kang et al. [13], and Patel et al. [14], highlighting the limited specificity of preoperative Tg in differentiating malignant from benign thyroid conditions. The high Tg levels observed in both malignant and benign cases suggest that, although Tg may provide supplementary information in certain clinical contexts, it lacks sufficient diagnostic accuracy to be relied upon as a standalone marker for malignancy in the preoperative setting. These findings support the conclusion that preoperative Tg testing alone is unreliable for distinguishing between benign and malignant thyroid nodules.
A key strength of the study is the inclusion of a relatively large sample size from a single tertiary center with standardized surgical and pathological protocols, ensuring consistency in data collection and analysis. Additionally, excluding cases with incomplete records enhanced data reliability, and comparing cytological, radiological, and histopathological findings enabled a comprehensive assessment.
However, several limitations should be acknowledged. The retrospective design inherently carries a risk of selection and information bias. As a single-center study, the findings may not be generalizable to other populations or institutions with differing referral patterns or laboratory assays. Furthermore, thyroglobulin levels above 500 ng/mL could not be quantified precisely due to the assay’s upper limit, which may have limited the ability to explore potential dose response relationships. Finally, the absence of long-term postoperative follow-up data precluded evaluation of the prognostic significance of preoperative thyroglobulin levels.
Conclusion
Preoperative Tg levels may be elevated in both benign and malignant thyroid disorders; however, Tg may not possess adequate diagnostic precision to be used as a sole indicator of malignancy.
Declarations
Conflicts of interest: The authors have no conflicts of interest to disclose.
Ethical approval: Ethical approval was obtained from the “Kscien Organization’s Ethics Committee under registration number 40 in 2025.
Consent for participation: Not applicable.
Consent for publication: Not applicable.
Funding: The present study received no financial support.
Acknowledgements: None to be declared.
Authors' contributions: FHK, HAN, and AMS: Major contributors to the conception and design of the study, supervision of the project, and drafting and critical revision of the manuscript for important intellectual content. SFA: Contributes to data analysis and interpretation and participates in manuscript revision. ReMA, RaMA, and AMA: Involve in study design and assist with initial drafting and revision of the manuscript. HOB, ROM, ANQ, SHH, AAQ, HAA, and BAA: Contribute to data acquisition and collection. All authors read and approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Use of AI: ChatGPT-3.5 was used to assist with language refinement and improve the overall clarity of the manuscript. All content was thoroughly reviewed and approved by the authors, who bear full responsibility for the final version.
Data availability statement: Not applicable.
Atypical Presentations of Pilonidal Sinus Disease: A Case Series with Literature Review
Shaban Latif, Hemn H. Kaka Ali, Deari A. Ismaeil, Karzan M. Salih, Ayman M. Mustafa, Shko H....
Abstract
Introduction: Pilonidal sinus (PNS) typically arises in the sacrococcygeal region but can occasionally present in atypical locations, including the axilla, intermammary region, umbilicus, interdigital web, and neck, often leading to delayed diagnosis and suboptimal management. This study aims to examine clinical features, surgical management, and outcomes of PNS in these uncommon sites.
Methods: This retrospective case series was conducted at a single center over eight years and examined patients with extra-sacrococcygeal PNS confirmed by histopathology. Clinical, surgical, and follow-up data were retrieved from patient records. Data were analyzed using SPSS 25.0 and presented as descriptive statistics (frequencies, percentages, means, and ranges).
Results: This study included 4 patients, with an equal sex distribution (2 females, 50%; 2 males, 50%) and a mean age of 24 years (range: 16–31). Presenting symptoms included discharge, itching, pain, redness, and mass formation. None had significant past medical history, and one patient (25%) had prior surgery at the same site. Affected sites were equally distributed: breast (25%), axilla (25%), penile (25%), and scalp (25%). All patients underwent surgical intervention, and histopathology revealed tracts lined with epidermis, surrounded by mixed inflammatory cells, foreign body giant cells, and hair shafts in granulation tissue. During the follow-up period (mean follow-up: 13.5 months), no recurrence was observed.
Conclusion: Atypical presentations of PNS, although rare, can occur in diverse anatomical regions. Surgical excision with primary closure might be an effective treatment approach, potentially leading to favorable clinical outcomes and a low recurrence rate.
Introduction
Pilonidal sinus (PNS) is a common inflammatory disorder that typically occurs in the natal cleft, resulting from hair penetration into the skin, forming a tract lined with granulation tissue, which may develop into a pus-filled cavity [1,2]. The condition was first described by Mayo in 1833, who identified a hair-containing cyst just below the coccyx. The term "pilonidal" was coined by Hodge in 1880 and is derived from its Latin roots. Despite being recognized for nearly two centuries, the pathogenesis of PNS remains a subject of ongoing debate [3]. Notable contributions by Karydakis and Bascom have shifted the understanding of PNS from a congenital neural tube abnormality to an acquired condition, emphasizing the role of environmental and lifestyle factors [4,5]. PNS risk factors include poor hygiene, a deep natal cleft, excessive hair growth, and a sedentary lifestyle. Although often asymptomatic, PNS may present as a recurrent abscess or a non-healing draining sinus [4]. The condition predominantly affects the sacrococcygeal area of hirsute males, with an estimated incidence of 26 per 100,000 people [6]. However, in rare cases, PNS can develop in atypical locations, including the axilla, intermammary region, umbilicus, interdigital webs, occiput, scalp, endoanal canal, groin, suprapubic area, clitoris, penis, and prepuce [2,3,7].
Given the scarcity of literature on atypical presentations of PNS, the current study aims to examine the clinical features and management of PNS in atypical locations (extra-sacrococcygeal). The eligibility of the references has been confirmed [8].
Methods
Study design
This retrospective case series was conducted over eight years at a single private facility, focusing on patients with atypical PNS who were evaluated, treated, and followed up. Ethical approval for this study was obtained from the Ethical Committee of the Kscien Organization (Approval No. 31/2025). Clinical, surgical, and follow-up data were collected through a systematic review of electronic medical records, surgical pathology reports, and consultation with treating healthcare providers when necessary. Informed consent was obtained from all patients or their guardians for the publication of their anonymized data.
Setting and Participants
The study included patients with atypical (extra-sacrococcygeal) PNS presentations who were admitted to the hospital during the study period. Clinical and sociodemographic data were collected from patient records. The following variables were recorded: age, gender, signs and symptoms, past medical and surgical history, clinical examination findings, ultrasound (US) findings, surgical indications, type of surgery performed, histopathological results, complications, and follow-up duration.
Inclusion and exclusion criteria
Eligible participants were those with confirmed PNS diagnoses through HPE. Patients with incomplete clinical or sociodemographic data or those whose diagnosis could not be confirmed through histopathology were excluded. Patients with primary sacrococcygeal PNS or those lacking follow-up data were also excluded from the study.
Surgical Management
All patients underwent thorough preoperative planning and assessments to evaluate their suitability for local anesthesia. Preoperative evaluations included US, monitoring of vital signs, viral screening, and hematological assessment, including a complete blood count and hemoglobin level measurement. Surgical procedures were performed with patients in the supine position. The surgical approach involved wide local excision of the PNS followed by primary closure. Following wide local excision, Salih’s preparation (a mixture of sclerosing agent and Lawsonia inermis) was applied to the wound bed and sinus margins prior to primary closure. This was performed to enhance wound healing and reduce the risk of infection [9]. Postoperative pain was managed with oral paracetamol at a dose of 1,000 mg, administered every 6–8 hours as needed, for a minimum of five days.
Statistical analysis
The data were organized and analyzed using Microsoft Excel 2019. Descriptive analysis was conducted using the Statistical Package for Social Sciences (SPSS) version 25.0. The results are presented as frequencies, percentages, means, and ranges.
Results
Clinical and Histopathological Features
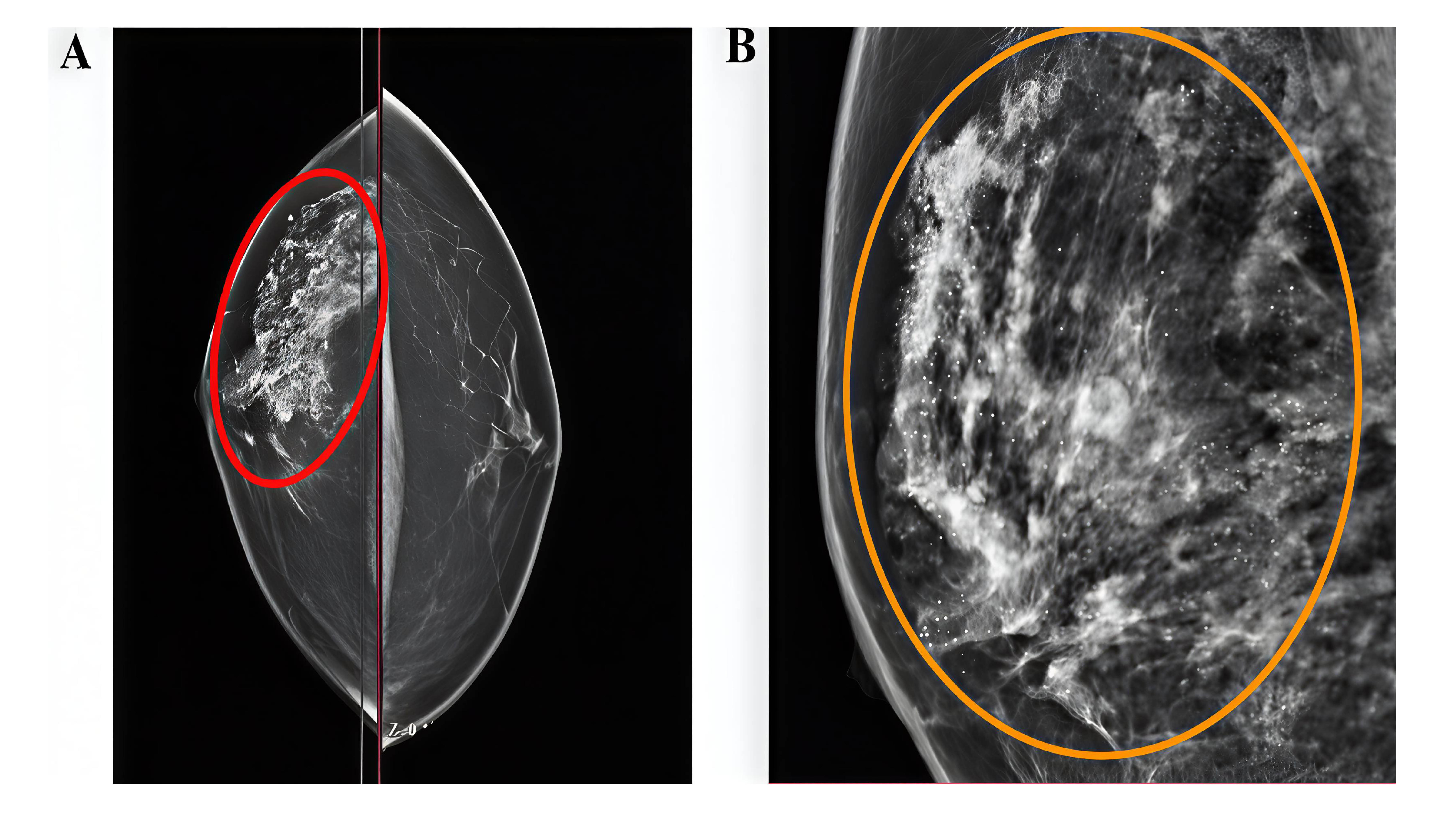
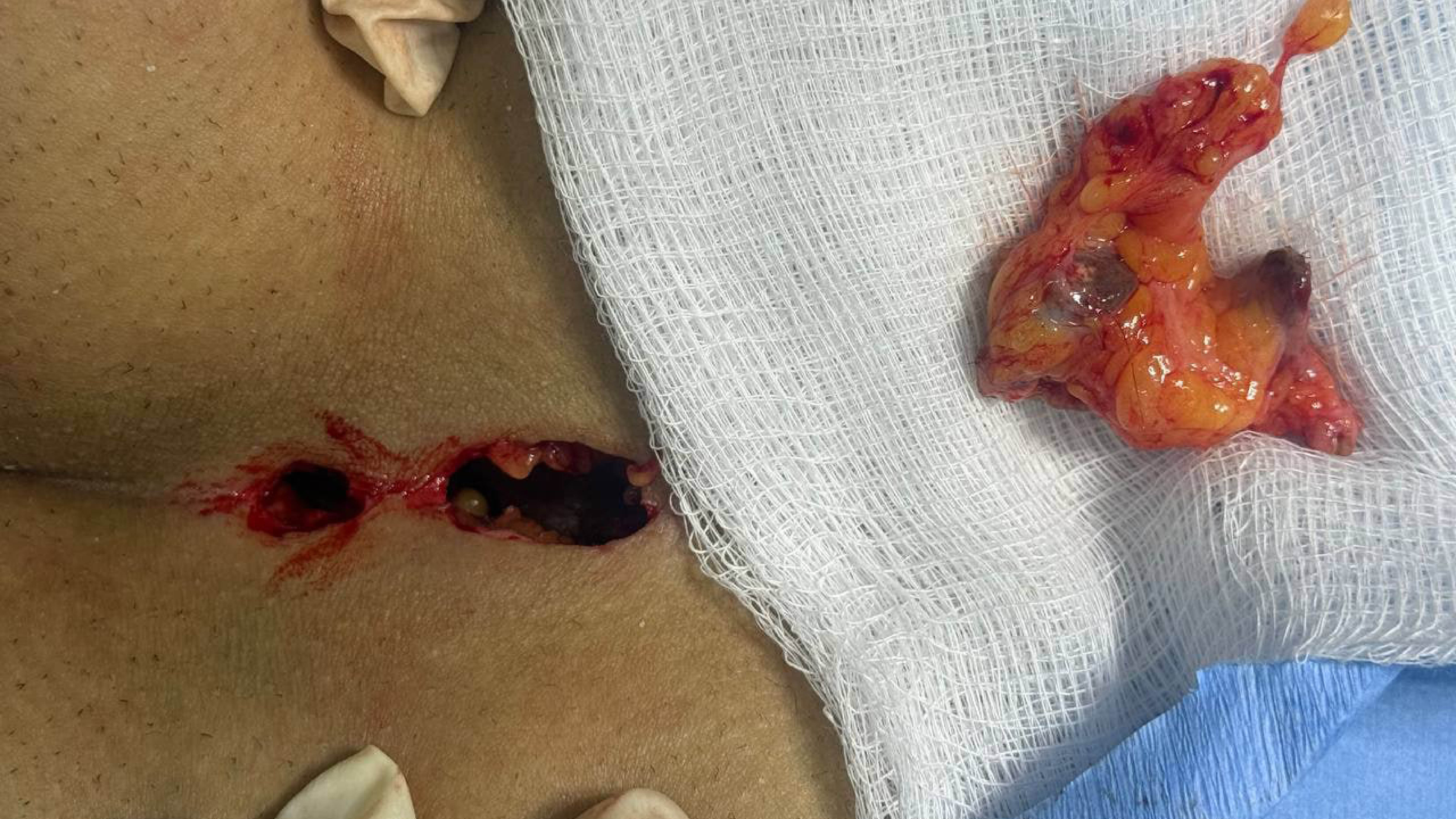
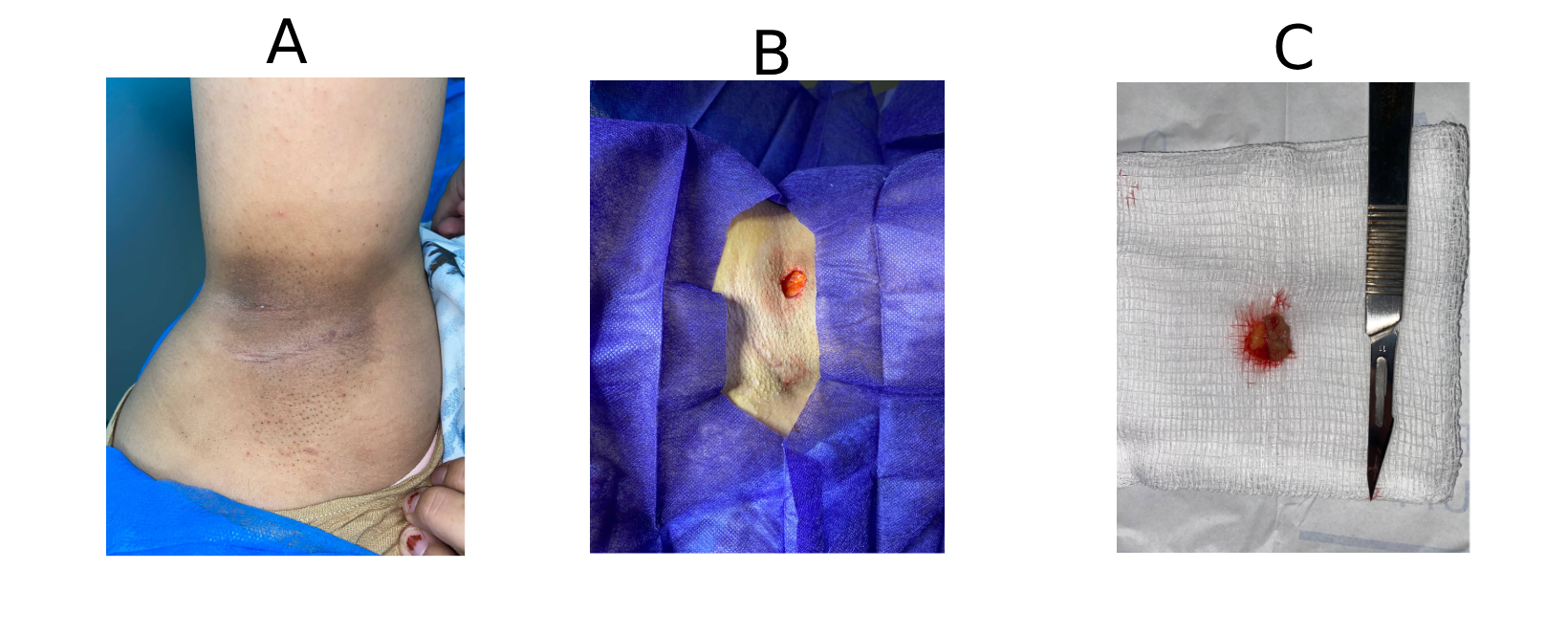
This study included four patients, comprising 2 females (50%) and 2 males (50%), with a mean age of 24 years (range: 16–31 years) (Table 1). Patients presented with various symptoms, including discharge, itching, pain, and erythema. None of the patients had significant past medical history. One patient (25.0%) had a history of prior surgery at the same site. The affected sites varied, with the breast, axilla, penile, and scalp region each in one case (Figures 1 and 2). Ultrasound imaging was performed in three of four cases (75%), and the findings revealed simple breast cysts with associated midline and inframammary PNS, infected dermal–subdermal cysts with mild edema, and chronic PNS in the left temporal scalp region (Table 1). All patients underwent surgical intervention, including excision of sinuses or complete removal of masses/nodules. HPE revealed tracts lined with epidermis, surrounded by mixed inflammatory cells, foreign body giant cells, and hair shafts embedded in granulation tissue.
|
Case / Authors [Ref] |
Age (Y) |
Sex |
Sign & Symptoms |
Location |
PMH |
PSH |
Examination Findings |
Imaging (US) |
Surgical Indication |
Type of Surgery |
||
|
Salih & Kakamad [2] |
39 |
M |
Perianal discharge and pruritus |
Endoanal |
Negative |
Surgically treated PNS and anal fistula |
External opening at 11 o’clock, 3.5 cm from anal verge with induration and surrounding excoriation |
Single fistulous tract extending into anal canal |
Discharge and pain during defecation |
Fistulectomy |
||
|
Salih & Kakamad [3] |
19 |
M |
Chronic nodule |
Scalp |
Negative |
Negative |
2×2 cm mobile nodule in left parieto-occipital region with central punctum |
Not performed |
Intermittent discharge |
Complete excision |
||
|
Salih et al. [7] |
22 |
F |
Painful submental mass |
Submental |
Negative |
Operation at same site 6 years prior |
Tender 2×2 cm mass attached to skin, no external opening |
Well-defined hyperechoic lesion suggestive of dermoid cyst |
Painful mass |
Complete excision |
||
|
Salih et al. [10] |
27 |
M |
Painless postauricular discharge |
Auricle |
Negative |
Negative |
NA |
Not performed |
Discharge |
Excision of sinuses |
||
|
Salih et al. [11] |
25 |
F |
Bilateral Multiple discharging sinuses |
Breast |
Negative |
Negative |
Multiple discharging sinuses with induration and tenderness |
Not performed |
Discharge |
Excision of sinuses |
||
|
Salih & Kakamad [12] |
22 |
M |
Pain and purulent discharge |
Auricle |
Negative |
Negative |
2 cm scar with single central opening |
Not performed |
Pain and discharge |
Complete excision |
||
|
Salih et al. [13] |
20 |
F |
Yellowish discharge |
Neck |
Negative |
Negative |
10 openings on nape, 7×4 cm induration and erythema |
Not performed |
Multiple sinuses with discharge |
Excision of sinuses |
||
|
Salih et al. [14] |
35 |
F |
Discharging lesion |
Breast |
Negative |
Negative |
Local redness with multiple discharging sinuses, 3–4 cm from nipple |
Not performed |
Remission and relapse |
Excision of sinuses |
||
|
Salih et al. [15] |
22 |
F |
Pain, discharge, and erythema |
Breast |
Negative |
Negative |
Single sinus with clear discharge, surrounding old scar |
Not performed |
Discharge |
Wide local excision |
||
|
Salih et al. [16] |
16 |
F |
Chronic discharging sinus |
Breast |
NA |
Negative |
Single discharging sinus, 8×9 cm induration |
Not performed |
Discharge |
Excision of sinuses |
||
|
Salih et al. [17] |
25 |
M |
Mass with chronic discharge |
Auricle |
Negative |
Negative |
1×1 cm firm, tender nodule attached to skin |
Not performed |
Discharge |
Mass excision |
||
|
Present study (Case 1) |
31 |
F |
Intermammary mass with erythematous nodules |
Breast |
Negative |
Negative |
Midline swelling without ulceration or discharge |
Simple breast cysts with midline and inframammary PNS |
Painful mass |
Complete excision |
||
|
Present study (Case 2) |
27 |
F |
Right axillary mass, 4-year duration |
Axilla |
Negative |
Negative |
Linear scar with mild hyperpigmentation, chronic PNS |
Infected dermal-subdermal cysts with mild edema |
Painful mass |
Excision of sinuses |
||
|
Present study (Case 3) |
22 |
M |
Perianal discharge and pruritus |
Penile |
Negative |
Negative |
Linear scar, chronic PNS without active inflammation |
NA |
Sinus |
Excision of sinus |
||
|
Present study (Case 4) |
16 |
M |
Painless scalp discharge |
Scalp |
Negative |
Skin laceration at same site |
NA |
Left temporal scalp chronic PNS |
Sinus |
Excision of sinus |
PNS |
No recurrence at 3 months |
|
F: Female, M: Male, PMH: past medical history, PSH: past surgical history, PNS: pilonidal sinus, US: ultrasound, NA: not available, Y: Years |
||||||||||||
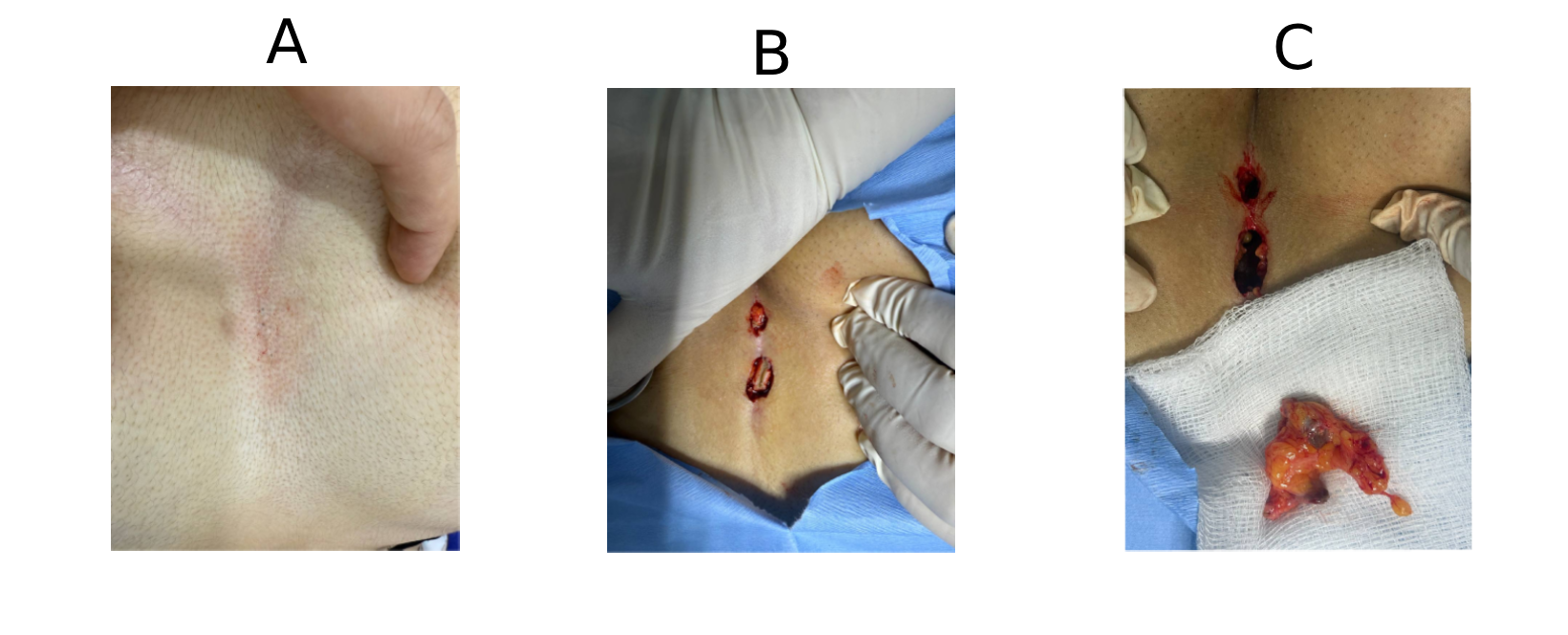
Outcomes and Follow-up
All 4 cases had favorable outcomes with no recurrence. Follow-up duration ranged from 1 month to 4 years, with the mean follow-up period being 13.5 months. No significant complications or adverse events occurred, and postoperative recovery was uneventful, with no need for further interventions.
Discussion
The pilonidal disease has a spectrum of clinical manifestations, ranging from asymptomatic to large, symptomatic abscesses. The exact cause of PNS remains unclear, with two main theories, acquired and congenital, offering potential explanations for its development. The formation of PNS generally requires three key factors: the presence of hair in the skin, a region of wrinkled skin (such as the natal cleft or a scar), and a combination of hormonal and hygiene-related factors [5]. This condition is less frequent before puberty and after the age of 40 years, likely due to the influence of sex hormones during these periods. It is also three times more common in males than females. Studies have shown that individuals who sit for prolonged periods and bathe less frequently are at a significantly higher risk of developing PNS, with a 219-fold increased risk compared to those without these risk factors [2,10]. Among the 11 cases reported in the literature, the mean patient age was 24.7 years (range: 16–39 years). The most commonly affected site was the breast (36.4%), followed by the auricle (27.3%), with single cases involving the endoanal, scalp, submental, and neck regions (each 9.1%). The sex distribution showed a slight male predominance, with 6 males (54.5%) and 5 females (45.5%) (Table 1) [2,3,7,10–17]. In line with these findings, the current case series included 4 patients with an equal sex distribution (2 males, 50%; 2 females, 50%) and a comparable mean age of 24 years (range: 16–31 years).
The differential diagnosis for atypical PNS includes a broad range of conditions, such as hernia, endometriosis, urachal cyst, pyogenic granuloma, epidermoid cyst, dermoid cyst, and infected sebaceous cyst. Clinical signs often include pain, warmth, and swelling, indicating inflammation. This condition is multifaceted, causing physical discomfort and emotional distress for those affected, leading to direct healthcare costs and indirect societal costs due to lost work hours [11,12]. Among the cases reviewed, the most common presenting symptom was discharge (9/11, 81.8%), followed by pain (3/11, 27.3%) and mass or nodule formation (2/11, 18.2%) [2,3,7,10–17]. In the current series, two (50%) presented with masses (intermammary and axillary), and two (50%) presented with discharge (perianal and scalp), with pruritus, erythema, painless presentation, and chronicity each observed in a single case (25%).
PNS is most commonly diagnosed based on the clinical presentation, with laboratory or radiologic investigations generally unnecessary [10]. However, US can reveal strands of hair associated with the lesion. Fine needle aspiration cytology can help to rule out a neoplastic process, particularly when the suspicion for PNS disease is low [10]. Ultrasonography was performed in only 2 of the 11 cases reviewed (18.2%). One case demonstrated a single fistulous tract extending into the anal canal, while another revealed a well-defined hyperechoic lesion suggestive of a dermoid cyst. In the remaining 9 cases (81.8%), ultrasound evaluation was not carried out [2,3,7,10–17]. In the current series, ultrasound was performed in three of four cases (75%). Findings included simple breast cysts with associated midline and inframammary PNS, infected dermal–subdermal cysts with mild edema, and chronic PNS in the left temporal scalp region
A variety of both surgical and non-surgical approaches are utilized in managing PNS. Surgical interventions include simple incision and drainage, marsupialization, open wound management, primary closure, and rhomboid excision followed by a Limberg flap. Non-surgical methods typically involve the injection of sclerosing and wound-enhancing agents into the sinus tract. Managing atypical PNS often necessitates more tailored approaches, such as resection with or without primary closure. Postoperative complications and recurrences are common, with significant risk factors identified, including obesity, a family history of PNS, male sex, tobacco use, inadequate hygiene, sinus size, and the specific surgical technique employed [3, 4]. The ideal management of PNS should aim to alleviate patient discomfort, reduce hospital stays, minimize recurrence rates, address complications, and limit prolonged work absences [4].
Furthermore, the treatment should be safe, effective, and free from adverse consequences. Surgical approaches, however, may not align with these optimal treatment goals, as they can result in discomfort and prolonged work absences. While surgery remains the recommended approach for atypical PNS, it also facilitates a definitive diagnosis through HPE. Shareef et al. conducted a study involving 12 female patients, all presenting with discharge lasting from four weeks to two years. Nine of these patients underwent resection with direct closure, without a flap, while the remaining three had their PNS excised and allowed to heal secondarily. Recurrence was observed in three of the patients [13-18]. Among the cases reviewed, excision of sinuses was the most commonly performed procedure (5/11, 45.5%), followed by complete excision (3/11, 27.3%). Fistulectomy, wide local excision, and mass excision were each performed in a single case (9.1%) [2,3,7,10–17]. In the current series, surgical management included excision of a single sinus in two cases (50%), complete excision in one case (25%), and excision of multiple sinuses in one case (25%). All 15 cases, including 11 reviewed in the literature and 4 from the current series, remained symptom-free with no recurrence during follow-up.
This study has several limitations. First, the retrospective design may introduce selection bias and limit the ability to establish causal relationships. Second, the small sample size limits the generalizability of the findings. Third, the follow-up period varied among the patients, which could affect the assessment of long-term outcomes. Finally, the study was conducted at a single center, which may not reflect the broader population.
Conclusion
This case series highlights the rare occurrence of PNS in atypical locations, with clinical and HPE findings that resemble typical cases. Surgical excision might result in favorable outcomes without recurrence.
Declarations
Conflicts of interest: The authors have no conflicts of interest to disclose.
Ethical approval: Ethical approval was obtained from the Ethical Committee of the Kscien Organization (Approval No. 31/2025)
Consent for participation: Not applicable.
Consent for publication: Informed consent was obtained from all patients or their guardians for the publication of their anonymized data.
Funding: The present study received no financial support.
Acknowledgements: None to be declared.
Authors' contributions: SL, HHK, DAI, and AMM: Major contributors to the conception of the study, as well as the literature search for related studies, and manuscript writing. KMS, SHH, KAN, HAA, AAQ and AMA: Literature review, critical revision of the manuscript, and processing of the tables and figures. All authors have read and approved the final version of the manuscript.
Use of AI: ChatGPT-3.5 was used to assist with language refinement and improve the overall clarity of the manuscript. All content was thoroughly reviewed and approved by the authors, who bear full responsibility for the final version.
Data availability statement: Not applicable.