Pembrolizumab and Sarcoma: A meta-analysis
Abstract
Introduction: Pembrolizumab is a monoclonal antibody that promotes antitumor immunity. This study presents a systematic review and meta-analysis of the efficacy and safety profile of this treatment as monotherapy or in combination with other drugs for the treatment of sarcomas.
Methods: A literature search was conducted across Google Scholar, PubMed/MEDLINE, and EMBASE from February 15th to April 15th. Eligible studies were clinical trials that reported efficacy or outcomes of pembrolizumab in sarcoma patients, either alone or in combination with other drugs. In contrast, those lacking sufficient data or not meeting trial criteria were excluded.
Results: Ten clinical trials met the eligibility criteria, including 419 sarcoma patients (53.7% male; median age 55.4). Pembrolizumab was administered either as monotherapy in 23% of cases or in combination with other agents in 77% of cases. The progressive disease rate was 83% with monotherapy and 36% with combination therapy. Objective response rates varied, with the highest observed in the pembrolizumab plus talimogene laherparepvec combination (35%) and the lowest in pembrolizumab monotherapy (ranging from 0% to 11.2%). Median progression-free survival ranged from 1.4 (Pembrolizumab + Cyclophosphamide) to 7.8 months (Pembrolizumab + Lenvatinib in undifferentiated pleomorphic sarcoma). Combination therapy was associated with significantly better tumor response (<0.001). However, rates of endocrine, gastrointestinal, some hepatic, and dermatological adverse events were significantly associated with combination therapy compared to monotherapy (p < 0.05).
Conclusion: Pembrolizumab-based combination therapies have the potential to enhance treatment efficacy in sarcoma, although they may be associated with an increased risk of adverse events.
Introduction
Sarcomas are rare tumors that develop from mesenchymal tissue and are known for their diverse histological subtypes. Although they are uncommon, they carry particular clinical significance due to their relatively higher incidence in adolescents and young adults. The primary treatment is surgical resection, often combined with chemotherapy or radiotherapy, either before (neoadjuvant) or after (adjuvant) surgery, depending on the specific subtype [1].
Immunotherapy has introduced a breakthrough in cancer treatment. Unlike traditional approaches such as chemotherapy and radiation, which can harm healthy cells and lead to significant side effects, immunotherapy takes a more targeted and refined approach. It helps the body’s immune system recognize and attack cancer cells, offering a potentially more effective and less toxic alternative [2].
Cancer immunotherapy includes a variety of treatment methods, such as immune checkpoint inhibitors, adoptive cell transfer, cytokine therapies, and cancer vaccines [3]. What sets immunotherapy apart is its ability to produce long-lasting effects, sometimes leading to extended remission or even complete disappearance of the disease in some patients [4]. In addition, immunotherapy is often better tolerated than traditional treatments, since it tends to focus more precisely on cancer cells while causing less harm to healthy tissues [2].
Pembrolizumab is a monoclonal antibody that targets the programmed cell death protein 1 (PD-1) receptor, blocking its interaction with the ligands PD-L1 and PD-L2, which are often expressed by tumor cells. By interrupting this pathway, pembrolizumab removes a key mechanism of immune suppression, allowing cytotoxic T cells to become more active and better able to attack cancer cells [5]. PD-1 is an immune checkpoint receptor found on activated T cells. When it binds to PD-L1 or PD-L2, it downregulates immune activity by inhibiting T-cell function and helping tumor cells avoid destruction. Research has shown that tumors often increase PD-L1 expression as a strategy to escape immune detection, weakening the body’s ability to fight cancer [6].
Despite significant research, sarcomas remain difficult to treat effectively. Soft tissue sarcomas, in particular, have a 5-year survival rate of only about 65%, highlighting the ongoing need for more effective therapeutic options [1]. Pembrolizumab has shown durable antitumor activity across several solid tumor types, along with a generally favorable safety and tolerability profile [7]. This systematic review and meta-analysis aims to assess the antitumor efficacy and safety of pembrolizumab, both as a standalone therapy and in combination with other agents, in treating different types of sarcomas through a systematic review and meta-analysis of clinical trials. Only data from peer-reviewed sources were included, ensuring the reliability of the data [8].
Methods
Study design
The study is a systematic review and meta-analysis encompassing clinical trials assessing the effectiveness of pembrolizumab in treating sarcomas. It encompasses trials where pembrolizumab is used alone as well as in combination with other treatments. The drug combinations were as follows: group A (Pembrolizumab + Doxorubicin), group B (Pembrolizumab + Axitinib), group C (Pembrolizumab + Eribulin), group D (Pembrolizumab + Talimogene Laherparepvec), group E (Pembrolizumab + Epacadostat), group F (Pembrolizumab + Olaratumab), group G (Pembrolizumab + Levatinib), and group H (Pembrolizumab + Cyclophosphamide). The search process was conducted from February 15th to April 15th 2025, in full compliance with the PRISMA 2020 (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.
Data sources and search strategy
A thorough search was performed using Google Scholar, PubMed/MEDLINE, and EMBASE. A set of keywords was utilized to retrieve pertinent studies, specifically: (anti-PD-1; PD-1 inhibitor; Keytruda; MK-3475; Lambrolizumab; Pembrolizumab; Sarcoma; Sarcomas; Spindle cell sarcoma; Spindle cell sarcomas).
Eligibility criteria
Eligible studies consisted of clinical trials evaluating the efficacy of pembrolizumab in treating sarcomas, either as a standalone therapy or in combination with other treatments. Studies were excluded if they were not clinical trials, did not investigate pembrolizumab for sarcoma treatment, or failed to provide adequate data on treatment efficacy or patient outcomes.
Study selection process
The study selection process was conducted independently by two researchers who carefully screened the titles and abstracts of all identified studies. Each study was assessed based on the predefined inclusion and exclusion criteria. In instances of disagreement regarding a study’s eligibility, a third researcher was consulted to resolve the conflict and reach a consensus.
Data items
Data extracted from the eligible studies encompassed a wide range of information, including the first author’s name, year of publication, trial phase, sarcoma subtype, number of enrolled patients, sex distribution, treatment regimen, median patient age, reported adverse events, and various clinical outcome measures, including progression-free survival, overall survival, complete response, partial response, stable disease and progressive disease.
Data analysis and synthesis
The extracted data were organized using Microsoft Excel (2019) and analyzed with SPSS version 25.0 (IBM Corp.). Descriptive statistics were reported as frequencies, percentages, medians, and ranges. Categorical variables were compared using the Chi-square or Fisher's exact test, as appropriate. Continuous variables, including overall survival and progression-free survival (PFS), were analyzed using the non-parametric Mann-Whitney U test.
Results
Study selection process and eligibility criteria
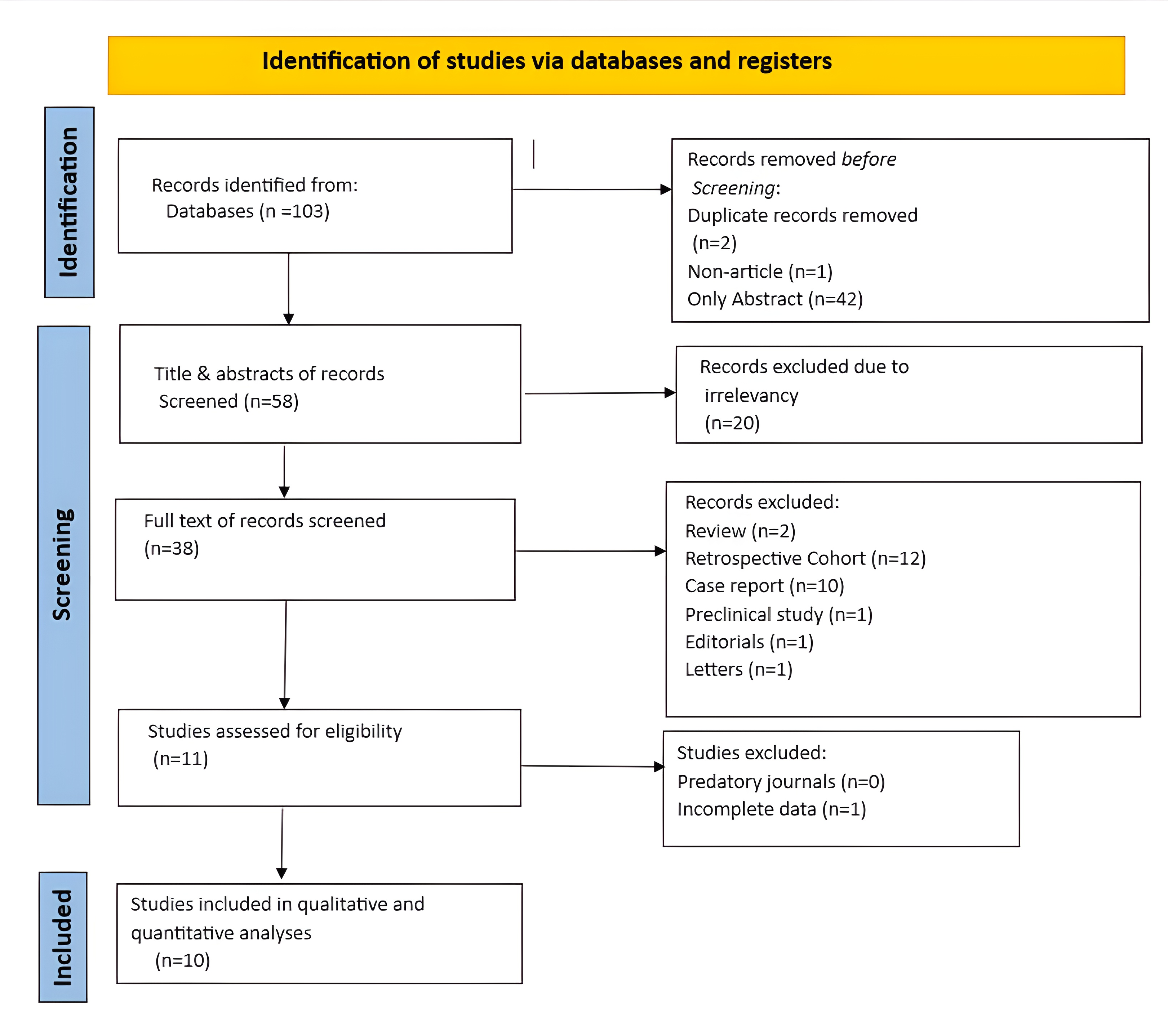
A total of 103 records were initially identified through the search process. Among these, 42 were excluded because they were unretrievable, two were removed as duplicates, and one was excluded for not qualifying as a full research article. Then, the titles and abstracts of the remaining 58 studies were screened, resulting in the exclusion of 20 records that were deemed irrelevant. After that, 38 studies underwent full-text screening, of which 27 were excluded based on exclusion criteria. Subsequently, 11 studies were assessed for eligibility, and one was excluded because it did not provide complete data. In the end, 10 studies were included for final review and analysis (Figure 1) [7, 9-17].
Characteristics of included trials
All the studies included were clinical trials, consisting of eight Phase II trials, one Phase I trial, and one study that encompassed both Phase I and II designs. The raw data, along with key characteristics of each study, are summarized in Tables 1, 2, and 3. Analysis of progressive disease rates in sarcoma patients treated with pembrolizumab monotherapy, based on two studies, revealed an overall rate of 83% (95% CI: 26%–100%). In contrast, a meta-analysis of patients receiving pembrolizumab in combination with other therapeutic agents showed a substantially lower progressive disease rate of 36% (95% CI: 23%–51%) (Figure 2).
![Figure 2. Forest plot of studies evaluating progressive disease rates with (A) Pembrolizumab monotherapy vs. (B) Pembrolizumab combination therapies*. CI, confidence interval *Olaratumab combination trial was excluded due to unavailable progressive disease data [16].](https://www.judijournal.com/public/site/images/journaleditor/mceu-5288121221751108819164.png)
|
First Author /Year [reference] |
Trial Phase |
Type of sarcoma |
Therapy regimens
|
No. of patients |
Sex |
Median Age |
|
|
Male |
Female |
||||||
|
Pollack et al,2020 [10] |
I/II |
Soft tissue sarcoma |
Pembrolizumab 200mg/q3w(IV) Doxorubicin 45-75mg |
37 |
22 |
15 |
58.4 |
|
Wilky et al, 2019 [11] |
II |
Soft tissue sarcoma |
Pembrolizumab 200mg/q3w (IV) Axitinib 5mg (Orally) |
33 |
18 |
15 |
44 |
|
Tawbi et al,2017 [9] |
II |
Soft tissue sarcoma + Osteosarcoma |
Pembrolizumab 200mg/q3w (IV) |
84 |
53 |
31 |
53a/ 33b |
|
Haddox et al,2024 [17] |
II |
Soft tissue sarcoma |
Pembrolizumab 200mg/q3w(IV) Eribulin 1.4mg/m2 (iv) |
57 |
24 |
33 |
60.4* |
|
Kelly et al,2020 [13] |
II |
Soft tissue sarcoma |
Pembrolizumab 200mg/q3w(IV) Talimogene Laherparepvec ≤4ml×102 (intratumourally) |
20 |
12 |
8 |
58.3 |
|
Kelly et al, 2023 [14] |
II |
Soft tissue sarcoma |
Pembrolizumab 200mg/q3w (IV) Epacadostat 100mg (orally) |
30 |
18 |
12 |
54 |
|
Schöffski et al, 2023 [16] |
Ia/b |
Soft tissue sarcoma |
Pembrolizumab 200mg/q3w(IV) Olaratumab 15-20mg/kg (IV) |
41 |
15 |
26 |
56.83* |
|
Movva et al,2024 [12] |
II |
Soft tissue sarcoma + Bone sarcoma |
Pembrolizumab 200mg/q3w(IV) Levatinib 20mg (orally) |
48 |
22 |
26 |
50 |
|
Toulmonde et al, 2018 [15] |
II |
Soft tissue sarcoma |
Pembrolizumab 200mg/q3w (IV) Cyclophosphamide 50mg (Orally) |
57 |
33 |
24 |
59.5 |
|
Boye et al,2021 [7] |
II |
Osteosarcoma |
Pembrolizumab 200mg/q3w (IV) |
12 |
8 |
4 |
43 |
|
*: mean age, a: median age in soft tissue sarcoma, b: median age in bone sarcoma, q3w: every three weeks, IV: intravenous |
|||||||
|
First Author /Year [reference] |
Patient No. (Evaluable for response) |
Prior chemotherapy |
Therapy Regimen |
Outcome |
ORR (%) |
|||||||
|
Yes |
No |
CR |
PR |
SD |
PD |
NE |
||||||
|
Pollack et al,2020 [10] |
37 |
9 |
28 |
Group A |
0 |
7 |
22 |
7 |
1 |
|
||
|
Wilky et al, 2019 [11] |
30 |
27 |
6 |
Group B |
0 |
8 |
9 |
13 |
3 |
|
||
|
Tawbi et al,2017 [9] |
80 |
80 |
0 |
Pembrolizumab alone |
1 |
8 |
24 |
47 |
4 |
11.2
|
||
|
Haddox et al,2024 [17] |
56 |
2.5(1.5)a |
0 |
Group C |
1b |
10
|
22 |
23 |
1 |
19.3
|
||
|
Kelly et al,2020 [13] |
20 |
19 |
1 |
Group D |
0 |
7 |
7 |
6 |
0 |
35
|
||
|
Kelly et al, 2023 [14] |
30 |
24 |
6 |
Group E |
0 |
1 |
13 |
16 |
0 |
3.3
|
||
|
Schöffski et al, 2023 [16] |
41 |
37 |
0 |
Group F |
0 |
6 |
N/A |
N/A |
N/A |
14.6
|
||
|
Movva et al,2024 [12] |
46 |
43 |
5 |
Group G |
0 |
8 |
31 |
7 |
2 |
15.2
|
||
|
Toulmonde et al, 2018 [15] |
48 |
55 |
2 |
Group H |
0 |
1 |
16 |
31 |
9 |
2
|
||
|
Boye et al,2021 [7] |
12 |
12 |
0 |
Pembrolizumab alone |
0 |
0 |
0 |
12 |
0 |
0
|
||
|
RECIST: Response Evaluation Criteria in Solid Tumors, ORR: Objective response rate, CR: complete response, PR: Partial response, SD: Stable disease, PD: progressive disease, NE: not evaluable. (a): mean (SD), (b) The patient had radiotherapy-associated angiosarcoma. |
||||||||||||
|
First Author /Year [reference] |
Patient No. |
Therapy Regimen |
RMD (months) |
Median follow-up (months) |
Median PFS (months) |
Median Overall survival (months) |
|
Pollack et al, 2020 [10] |
37 |
Group A |
N/A |
N/A |
8.1 |
27.6 |
|
Wilky et al, 2019 [11] |
33 |
Group B |
6.6 |
14.7 |
4.7 |
18.7 |
|
Tawbi et al, 2017 [9] |
84 |
Pembrolizumab alone |
7.6a/9.9b |
19.1a/17.8b |
4a/2b |
11.2a/12b |
|
Haddox et al, 2024 [17] |
57 |
Group C |
N/A |
14c/11.6d/7e |
2.5c/7.3d/2.9e
|
13.5c/22d/N/Ae |
|
Kelly et al, 2020 [13] |
20 |
Group D |
13 |
13 |
4 |
N/A |
|
Kelly et al, 2023 [14] |
30 |
Group E |
N/A |
29.7 |
1.7 |
16.9 |
|
Schöffski et al, 2023 [16] |
41 |
Group F |
16.2 |
N/A |
1.4f/2.8g/2.7h |
11.4f/16.4g/14.8h |
|
Movva et al, 2024 [12] |
48 |
Group G |
2.5 |
20 |
2.2c/5.7A/7.8B/6.4C/4.3D |
10.3A/N/AB/14.5C/13.8D/6.4E |
|
Toulmonde et al, 2018 [15] |
57 |
Group H |
N/A |
6.8 |
1.4 |
9.2 |
|
Boye et al, 2021 [7] |
12 |
Pemb. alone |
N/A |
N/A |
1.7 |
6.6 |
|
RMD: response median duration, PFS: progression-free survival, a: soft tissue sarcoma, b: bone sarcoma, c: Leiomyosarcoma, d: Liposarcoma, e: Undifferentiated pleomorphic sarcoma/other, f: Olaratumab plus Pembrolizumab, g: Olaratumab plus Pembrolizumab, h: Olaratumab Pembrolizumab, A: undifferentiated pleomorphic sarcoma, B: vascular sarcomas (angiosarcoma and epithelioid hemangioendothelioma), C: synovial sarcoma or malignant peripheral nerve sheath tumor, D: bone sarcomas (osteosarcoma and chondrosarcoma). |
||||||
Baseline characteristics and treatment group distribution
A total of 419 patients were included across the eligible studies, of which 225 (53.7%) were males. The median age (IQR) of the patients was 55.4 (14.4). Most patients had an Eastern Cooperative Oncology Group (ECOG) status of zero (156, 37.23%), followed by a score of one (139, 33.17%). Performance status was not reported for 124 patients (29.6%). Histologically, leiomyosarcoma was the most frequently reported subtype, accounting for 99 cases (25.85%), whereas conventional chondrosarcoma was the least common, identified in only three cases (0.78%). Three hundred twenty-nine (78.52%) patients exhibited both metastatic and locally advanced properties. Patients were categorized into two groups: 96 patients (23%) received pembrolizumab as a monotherapy, while 323 patients (77%) were treated with pembrolizumab in combination with other therapies. The types of sarcoma included in the trials were soft tissue sarcoma (354, 84.5%) and bone sarcoma (65, 15.5%) (Table 4).
|
Variables |
Frequency/percentage |
|
Median age (IQR) |
55.4 (14.4) |
|
Sex Male Female |
225 (53.7%) 194 (46.3%) |
|
ECOG Status ECOG status (0) ECOG status (1) Not mentioned |
156 (37.23%) 139 (33.17%) 124 (29.6%) |
|
Sarcoma types Soft tissue sarcoma Bone sarcoma |
354 (84.5%) 65 (15.5%) |
|
Histological subtypes Angiosarcoma Synovial sarcoma Dedifferentiated liposarcoma Pleomorphic liposarcoma Leiomyosarcoma Undifferentiated pleomorphic sarcoma Gastrointestinal stromal tumor Alveolar soft-part sarcoma Osteosarcoma Dedifferentiated chondrosarcoma Ewing sarcoma Conventional chondrosarcoma Others |
Evaluable patients (383) 17 (4.44%) 22 (5.74%) 40 (10.44%) 4 (1.04%) 99 (25.85%) 63 (16.45%) 11 (2.87%) 13 (3.39%) 36 (9.4%) 10 (2.61%) 15 (3.92%) 3 (0.79%) 50 (13.05%) |
|
Sarcoma stage Locally advanced Metastatic Both |
11 (2.63%) 79 (18.85%) 329 (78.52%) |
|
Treatment group Pembrolizumab alone Pembrolizumab combination |
96 (23.0%) 323 (77.0%) |
Objective response rate (ORR) was 19% for patients treated with pembrolizumab plus doxorubicin, 25% for pembrolizumab plus axitinib, and 11.2% for pembrolizumab alone in a cohort of 80 patients. Pembrolizumab plus eribulin yielded an ORR of 19.3%, while the combination with talimogene laherparepvec had the highest ORR at 35%. In contrast, pembrolizumab plus epacadostat showed a low ORR of 3.3%, and pembrolizumab plus olaratumab achieved an ORR of 14.6% (Table 2).
Response median duration was available for five trials and ranged from 6.6 to 13 months. Median progression-free survival ranged from 1.4 months to 7.8 months, and median overall survival ranged from 6.6 to 27.6 months (Table 3).
Efficacy and adverse events in different treatment groups
Anemia affected 30% of patients overall. It was persistent with Group C (52.6%) and Group D (30%). Lymphopenia was notable in pembrolizumab monotherapy (27%). Vomiting and diarrhea were most frequent with Group B (66.6%) and (57.5%), respectively. Hypothyroidism was high in Group B (63.6%) and Group G (29.1%). Elevated liver enzymes (ALP, AST, ALT) were widespread, especially with Group G (89.5%) and Group A (59.3%). Cough and dyspnea were seen across most regimens, but cough was particularly high with Group B (33%). Headache was a relatively common complaint, notably with Group F (24.3%). Cardiac events were rare, but hypertension was notable in Group B (48.4%) and Group G (56%). Alopecia was most common in Group A (42.4%) and Group C (24.5%). Fatigue was the most common general adverse event, affecting more than half of all patients and reaching up to 80% in Group D (Table 5).
|
Adverse events |
Total (%)* |
Pemb. (96)** |
G. A (37) |
G. B (33) |
G. C (57) |
G. D (20) |
G. E (30) |
G. F (41) |
G. G (48) |
G. H (57) |
|
Hematological |
|
|
|
|
|
|
|
|
|
|
|
Thrombocytopenia |
19 (4.5%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
1 (5%) |
1 (3.3%) |
0 (0.0%) |
17 (35.4%) |
0 (0.0%) |
|
Lymphopenia |
36 (8.6%) |
26 (27.0%) |
2 (5.4%) |
0 (0.0%) |
3 (5.2%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
5 (8.7%) |
|
Febrile neutropenia |
13 (3.1%) |
4 (4.1%) |
3 (8.1%) |
0 (0.0%) |
6 (10.5%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
|
Anemia |
126 (30.0%) |
43 (10.2%) |
5 (13.5%) |
0 (0.0%) |
30 (52.6%) |
6 (30%) |
6 (20%) |
11 (26.8%) |
13 (27%) |
12 (21%) |
|
Gastrointestinal |
|
|
|
|
|
|
|
|
|
|
|
Nausea |
138 (33.0%) |
22 (23.0%) |
32 (86.4%) |
22 (3.0%) |
25 (43.8%) |
6 (30%) |
4 (13.3%) |
6 (14.6%) |
15 (31.2%) |
6 (10.5%) |
|
Vomiting |
56 (13.4%) |
11 (11.4%) |
11 (29.7%) |
22 (66.6%) |
6 (10.5%) |
4 (20%) |
2 (6.6%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
|
Anorexia |
72 (17.2%) |
14 (14.5%) |
18 (48.6%) |
12 (36.3%) |
24 (42.1%) |
0 (0.0%) |
4 (13.3%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
|
Constipation |
55 (13.1%) |
17 (17.7%) |
5 (13.5%) |
9 (27.2%) |
15 (26.3%) |
0 (0.0%) |
0 (0.0%) |
9 (22%) |
0 (0.0%) |
0 (0.0%) |
|
Diarrhea |
96 (23.0%) |
15 (15.6%) |
8 (21.6%) |
19 (57.5%) |
12 (21.0%) |
0 (0.0%) |
2 (6.6%) |
12 (29.2%) |
21 (4.16) |
7 (12.2%) |
|
Decreased appetite |
7 (1.7%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
7 (17.1%) |
0 (0.0%) |
0 (0.0%) |
|
Xerostomia |
26 (6.2%) |
0 (0.0%) |
11 (29.7%) |
0 (0.0%) |
8 (14.0%) |
0 (0.0%) |
4 (13.3%) |
0 (0.0%) |
0 (0.0%) |
3 (5.2%) |
|
Abdominal pain |
27 (6.4%) |
13 (13.5%) |
2 (5.4%) |
0 (0.0%) |
3 (5.2%) |
0 (0.0%) |
1 (3.3%) |
8 (19.5%) |
0 (0.0%) |
0 (0.0%) |
|
Colitis |
5 (1.2%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
3(5.2%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
2 (4.2%) |
0 (0.0%) |
|
Colonic perforation |
1 (0.2%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
1 (2.1%) |
0 (0.0%) |
|
Endocrine |
|
|
|
|
|
|
|
|
|
|
|
Hypothyroidism |
61 (14.6%) |
2 (2.1%) |
7 (18.9%) |
21 (63.6%) |
5 (8.7%) |
4 (20%) |
4 (13.3%) |
0 (0.0%) |
14 (29.1%) |
4 (7%) |
|
Hyperthyroidism |
18 (4.3%) |
7 (7.2%) |
3 (8.1%) |
0 (0.0%) |
4 (7.0%) |
0 (0.0%) |
2 (6.6%) |
0 (0.0%) |
0 (0.0%) |
2 (3.5%) |
|
Hot flushes |
3 (0.7%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
1 (3.3%) |
0 (0.0%) |
0 (0.0%) |
2 (3.5%) |
|
Hepatobiliary |
|
|
|
|
|
|
|
|
|
|
|
Elevated ALP, AST, AP |
162 (38.7%) |
57 (59.3%) |
0 (0.0%) |
18 (1.6%) |
27 (47.3%) |
4 (20%) |
12 (40%) |
1 (2.4%) |
43 (89.5%) |
0 (0.0%) |
|
Increased bilirubin |
15 (3.6%) |
3 (3.1%) |
0 (0.0%) |
0 (0.0%) |
4 (7.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
8 (16.6%) |
0 (0.0%) |
|
Increased lipase |
28 (6.7%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
17 (29.8%) |
0 (0.0%) |
3 (10%) |
8 (19.5%) |
0 (0.0%) |
0 (0.0%) |
|
Increased serum amylase |
16 (3.8%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
11 (19.2%) |
3 (15%) |
2 (6.6%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
|
Respiratory |
|
|
|
|
|
|
|
|
|
|
|
Cough |
65 (15.5%) |
24 (25.0%) |
3 (8.1%) |
11 (33%) |
7 (12.2%) |
3 (15%) |
1 (3.3%) |
9 (22%) |
7 (14.6%) |
0 (0.0%) |
|
Dyspnea |
57 (13.6%) |
19 (19.8%) |
4 (10.8%) |
0 (0.0%) |
10 (17.5%) |
0 (0.0%) |
4 (13.3%) |
11 (26.8%) |
9 (18.7%) |
0 (0.0%) |
|
Hemoptysis |
7 (1.7%) |
0 (0.0%) |
4 (10.8%) |
3 (9.1%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
|
Nasopharyngeal congestion |
18 (4.3%) |
0 (0.0%) |
0 (0.0%) |
18 (54.5%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
|
Pneumothorax |
3 (0.7%) |
1 (1%) |
1 (2.7%) |
1 (3.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
|
Nervous system |
|
|
|
|
|
|
|
|
|
|
|
Vertigo |
1 (0.2%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
1 (3.3%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
|
Headache |
55 (13.1%)
|
9 (9.4%) |
4 (10.8%) |
5 (15.1%) |
7 (12.2%) |
0 (0.0%) |
2 (6.6%) |
10 (24.3%) |
18 (37.5%)
|
0 (0.0%) |
|
Cognitive disturbance |
5 (1.2%) |
4 (4.1%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
1 (2.0%) |
0 (0.0%) |
|
Seizure |
2 (0.4%) |
0 (0.0%) |
0 (0.0%) |
2 (6.1%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
|
Dysguisia |
22 (5.3%) |
0 (0.0%) |
10 (27%) |
0 (0.0%) |
12 (21.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
|
Cardiac-related |
|
|
|
|
|
|
|
|
|
|
|
Chest pain |
1 (0.2%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
1 (2.1%) |
0 (0.0%) |
|
Left ventricular dysfunction |
1 (0.2%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
1 (2.1%) |
0 (0.0%) |
|
Pericarditis |
1 (0.2%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
1 (2.1%) |
0 (0.0%) |
|
Cardiac arrest |
1 (0.2%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
1 (1.75%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
| Vascular-related | ||||||||||
|
Hypertension |
46 (11.0%) |
3 (3.1%) |
0 (0.0%) |
16 (48.4%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
27 (56%) |
0 (0.0%) |
|
Thromboembolic event |
7 (1.7%) |
4 (4.2%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
3 (6.2%) |
0 (0.0%) |
|
Dermatological |
|
|
|
|
|
|
|
|
|
|
|
Skin rash |
15 (3.6%) |
0 (0.0%) |
6 (16.2%) |
9 (27.2%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
|
Maculopapular rash |
40 (9.5%) |
4 (4.2%) |
4 (2.7%) |
0 (0.0%) |
12 (21.0%) |
0 (0.0%) |
9 (30%) |
0 (0.0%) |
11 (23%) |
0 (0.0%) |
|
Dry skin |
2 (0.4%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
2 (6.6%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
|
Pruritus |
37 (8.8%) |
6 (6.2%) |
9 (24.3%) |
9 (27.2%) |
6 (10.5%) |
4 (20%) |
1 (3.3%) |
0 (0.0%) |
0 (0.0%) |
2 (3.5%) |
|
Alopecia |
28 (6.7%) |
0 (0.0%) |
14 (42.4%) |
0 (0.0%) |
14 (24.5%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
|
Palmar-Plantar-erythrodysthesia syndrome |
10 (2.4%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
10 (21%) |
0 (0.0%) |
|
Metabolic and nutritional |
|
|
|
|
|
|
|
|
|
|
|
Hypoalbuminemia |
36 (8.6%) |
21 (21.8%) |
0 (0.0%) |
0 (0.0%) |
9 (0.0%) |
0 (0.0%) |
0 (0.0%) |
6 (14.6%) |
0 (0.0%) |
0 (0.0%) |
|
Hypokalemia |
24 (5.7%) |
11 (11.4%) |
0 (0.0%) |
0 (0.0%) |
7 (0.0%) |
0 (0.0%) |
0 (0.0%) |
6 (14.6%) |
0 (0.0%) |
0 (0.0%) |
|
Hypomagnesmia |
22 (5.2%) |
0 (0.0%) |
6 (16.2%) |
0 (0.0%) |
13 (0.0%) |
0 (0.0%) |
3 (10%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
|
Hypophosphatemia |
24 (5.7%) |
3 (3.1%) |
0 (0.0%) |
0 (0.0%) |
11 (0.0%) |
0 (0.0%) |
2 (6.6%) |
8 (19.5%) |
0 (0.0%) |
0 (0.0%) |
|
Hypertriglyceridemia |
3 (0.7%) |
0 (0.0%) |
0 (0.0%) |
3 (9.1%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
|
Investigations |
|
|
|
|
|
|
|
|
|
|
|
Decreased WBC count |
48 (11.4%)
|
0 (0.0%) |
3 (8.1%) |
0 (0.0%) |
31(54.3%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
14 (29.1%) |
0 (0.0%) |
|
Decreased neutrophil count |
47 (11.2%) |
0 (0.0%) |
9 (24%) |
0 (0.0%) |
32(56.1%) |
0 (0.0%) |
0 (0.0%) |
6 (14.6%) |
0 (0.0%) |
0 (0.0%) |
|
Elevated hemoglobin |
5 (1.2%) |
0 (0.0%) |
0 (0.0%) |
5 (15.0%) |
0(0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
|
Increased creatinine or BUN |
10 (2.4%) |
0 (0.0%) |
0 (0.0%) |
6 (18.1%) |
4(7.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
|
Decreased ejection fraction |
2 (0.4%) |
0 (0.0%) |
2 (5.4%) |
0 (0.0%) |
0(0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
|
MSK-Connective tissue-related |
|
|
|
|
|
|
|
|
|
|
|
Myalgia |
25 (6.0%) |
6 (6.2%) |
0 (0.0%) |
0 (0.0%) |
7 (12.2%) |
0 (0.0%) |
4 (13.3%) |
5 (12.1%) |
0 (0.0%) |
3 (5.2%) |
|
Arthralgia |
49 (11.6%) |
7 (7.3%) |
0 (0.0%) |
15 (45.4%) |
9 (15.7%) |
0 (0.0%) |
6 (20%) |
7 (17.1%) |
0 (0.0%) |
5 (8.7%) |
|
General |
|
|
|
|
|
|
|
|
|
|
|
Fatigue |
217 (51.8%) |
34 (35.4%) |
21 (56.7%) |
26 (78.7%) |
41 (72.0%) |
16 (80%) |
10 (33.3%) |
15 (36.5%) |
25 (52%) |
29(50.8%) |
|
Weight loss |
55 (13.1%) |
18 (18.7%) |
6 (16.2%) |
12 (36.3%) |
17 (29.8%) |
0 (0.0%) |
2 (6.6%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
|
Fever |
34 (8.1%) |
0 (0.0%) |
7 (19%) |
0 (0.0%) |
14 (24.5%) |
0 (0.0%) |
9 (30%) |
2 (4.8%) |
0 (0.0%) |
2 (3.5%) |
|
Insomnia |
13 (3.1%) |
7 (7.3%) |
0 (0.0%) |
0 (0.0%) |
3 (5.2%) |
1 (5%) |
2 (6.6%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
|
Tumor pain |
52 (12.4%) |
1 (1.0%) |
0 (0.0%) |
15 (45.4%) |
6 (10.5%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
30(52.6%) |
|
Ocular |
|
|
|
|
|
|
|
|
|
|
|
Dry eye |
8 (2.0%) |
0 (0.0%) |
8 (21.6%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0(0.0%) |
|
Optic neuritis/uveitis |
1 (0.2%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
1 (3.3%) |
0 (0.0%) |
0 (0.0%) |
0(0.0%) |
|
Infection |
|
|
|
|
|
|
|
|
|
|
|
Oral mucositis |
52 (12.4%) |
0 (0.0%) |
13 (35.1%) |
23 (69.6%) |
11 (19.2%) |
0 (0.0%) |
1 (3.3%) |
0 (0.0%) |
0 (0.0%) |
4(7.0%) |
|
Pericarditis |
1 (0.2%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
1 (2.1%) |
0(0.0%) |
|
Rectal or vaginal mucositis |
7 (1.7%) |
0 (0.0%) |
1 (2.7 %) |
6 (18.1%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0(0.0%) |
|
URTI |
4 (1.0%) |
0 (0.0%) |
4 (10.8%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0(0.0%) |
|
Immune-related |
|
|
|
|
|
|
|
|
|
|
|
Hyperglycemia |
34 (8.1%) |
17 (17.7%) |
0 (0.0%) |
9 (27.2%)
|
8 (14.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0(0.0%) |
|
Autoimmune colitis |
1 (0.2%) |
0 (0.0%) |
0 (0.0%) |
1 (3.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0(0.0%) |
|
Autoimmune hepatitis |
2 (0.4%) |
0 (0.0%) |
0 (0.0%) |
1 (3.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
1 (2.4%) |
0 (0.0%) |
0(0.0%) |
|
Autoimmune arthritis |
3 (0.7%) |
0 (0.0%) |
0 (0.0%) |
2 (6.0%) |
0 (0.0%) |
0 (0.0%) |
1 (3.3%) |
0 (0.0%) |
0 (0.0%) |
0(0.0%) |
|
Pulmonary embolism |
1 (0.2%) |
1 (1.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0(0.0%) |
|
Adrenal insufficiency |
1 (0.2%) |
1 (1.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0(0.0%) |
|
Pneumonitis |
10 (2.4%) |
2 (2.1%) |
0 (0.0%) |
0 (0.0%) |
2 (3.5%) |
4 (20%) |
2 (6.6%) |
0 (0.0%) |
0 (0.0%) |
0(0.0%) |
|
Interstitial nephritis |
1 (0.2%) |
1 (1.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0(0.0%) |
| * The percentages were calculated by dividing by the total number of enrolled patients (419), **The numbers between the parentheses represent the number of enrolled patients, URTI: Upper respiratory tract infection, Pemb: Pembrolizumab. | ||||||||||
Compared to pembrolizumab monotherapy, combination therapy was associated with significantly better tumor responses, including higher rates of partial and stable disease and a lower incidence of progressive disease (p < 0.001). Hematological toxicities differed significantly: thrombocytopenia was more frequent with combination therapy (p = 0.010), while lymphopenia and anemia were more common with monotherapy (p < 0.001 and p = 0.001, respectively). Gastrointestinal events such as nausea (p = 0.019), xerostomia (p = 0.001), and abdominal pain (p = 0.003) were also more common in combination therapy. Combination therapy was linked to higher rates of hypothyroidism (p < 0.001), hepatobiliary abnormalities, including elevated liver enzymes (p < 0.001), lipase (p = 0.001), and amylase (p = 0.029). Respiratory (cough: p = 0.006; rhinorrhea: p = 0.018), neurological (dysgeusia: p = 0.004), vascular (hypertension: p = 0.005), and dermatological toxicities (skin rash: p = 0.028; maculopapular rash: p = 0.047; alopecia: p = 0.001) were also significantly higher in combination therapy. Metabolic disturbances such as hypoalbuminemia (p < 0.001), hypokalemia (p = 0.011), and hypomagnesemia (p = 0.004) varied between groups. Additionally, adverse events such as decreased WBC and neutrophil counts, fatigue, tumor pain, and oral mucositis were more common in the combination group (p < 0.001), whereas insomnia (p = 0.014) and hyperglycemia (p < 0.001) were more frequent with monotherapy (Table 6).
|
Parameters |
Type of therapy |
P-value |
|
|
Pembrolizumab Alone |
Combination Therapy |
||
|
Progression-free survival, Days (Median, IQR) |
56.0 (75.0) |
103.5 (112.0) |
0.303 |
|
Overall survival, Days (Median, IQR) |
343 (201.0) |
417 (263.5) |
0.559 |
|
Outcome (N, %) * Complete response Partial response Stable disease Progressive disease |
1 (1.1) 8 (8.7) 24 (26.1) 59 (64.1) |
1 (0.4) 48 (17.6) 120 (44.1) 103 (37.9) |
<0.001 |
|
Hematological adverse events Thrombocytopenia Lymphopenia Febrile Neutropenia Anemia |
0 (0.0) 26 (27.1) 4 (4.2) 43 (44.8) |
19 (5.9) 10 (3.1) 9 (2.8) 83 (25.7) |
0.010 <0.001 0.506 0.001 |
|
Gastrointestinal adverse events Nausea Vomiting Anorexia Constipation Diarrhea Decreased Appetite Xerostomia Abdominal Pain Colitis |
22 (22.9) 11 (11.5) 14 (14.6) 17 (17.7) 15 (15.6) 0 (0.0) 0 (0.0) 13 (13.5) 0 (0.0) |
116 (35.9) 45 (13.9) 58 (18.0) 38 (11.8) 81 (25.1) 7 (2.2) 26 (8.0) 14 (4.3) 5 (1.5) |
0.019 0.611 0.538 0.167 0.054 0.359 0.001 0.003 0.593 |
|
Endocrine adverse events Hypothyroidism Hyperthyroidism |
2 (2.1) 7 (7.3) |
59 (18.3) 11 (3.4) |
<0.001 0.146 |
|
Hepatobiliary adverse events Elevated ALP, AST, ALT Elevated Bilirubin Elevated Lipase Elevated Serum Amylase |
3 (3.1) 0 (0.0) 0 (0.0) |
105 (32.5) 12 (3.7) 28 (8.7) 16 (5.0) |
<0.001 0.999 0.001 0.029 |
|
Respiratory adverse events Cough Dyspnea Hemoptysis Rhinorrhea Pneumothorax |
19 (19.8) 0 (0.0) 0 (0.0) 1 (1.0) |
41 (12.7) 38 (11.8) 7 (2.2) 18 (5.6) 2 (0.6) |
0.006 0.061 0.359 0.018 0.543 |
|
Neurological adverse events Headache Dysgeusia |
0 (0.0) |
46 (14.2) 22 (6.8) |
0.301 0.004 |
|
Vascular adverse events Hypertension |
|
43 (13.3) |
0.005 |
|
Dermatological adverse events Skin Rash Maculopapular Rash Dry Skin Pruritus Alopecia |
0 (0.0) 4 (4.2) 0 (0.0) 6 (6.3) 0 (0.0) |
15 (4.6) 36 (11.1) 2 (0.6) 31 (9.6) 28 (8.7) |
0.028 0.047 0.999 0.413 0.001 |
|
Metabolic and nutritional adverse events Hypoalbuminemia Hypokalemia Hypomagnesemia Hypophosphatemia |
21 (21.9) 11 (11.5) 0 (0.0) 3 (3.1) |
15 (4.6) 13 (4.0) 22 (6.8) 21 (6.5) |
<0.001 0.011 0.004 0.316 |
|
Musculoskeletal and connective tissue adverse events Myalgia Arthralgia |
6 (6.3) 7 (7.3) |
19 (5.9) 42 (13.0) |
0.811 0.149 |
|
Others Decreased WBC count Elevated HBG Decreased Neutrophil count Elevated Creatinine or BUN Fatigue Weight Loss Fever Insomnia Tumor pain Oral mucositis Rectal or Vaginal mucositis Hyperglycemia Pneumonitis |
0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 34 (35.4) 18 (18.8) 0 (0.0) 7 (7.3) 1 (1.0) 0 (0.0) 0 (0.0) 17 (17.7) 1 (1.0) |
48 (14.9) 5 (1.5) 47 (14.6) 10 (3.1) 183 (56.7) 37 (11.5) 34 (10.6) 6 (1.9) 51 (15.8) 52 (16.1) 6 (1.9) 17 (5.3) 8 (2.5) |
<0.001 0.593 <0.001 0.126 <0.001 0.084 <0.001 0.014 <0.001 <0.001 0.344 <0.001 0.69 |
|
* Analyses were done for evaluable cases, IQR: Interquartile range, ALP: Alkaline phosphatase, AST: Aspartate aminotransferase, ALT: Alanine Aminotransferase, WBC: White blood cells, HBG: Hemoglobin, BUN: Blood Urea Nitrogen |
|||
Discussion
The current meta-analysis results demonstrate a stark contrast between pembrolizumab monotherapy and combination approaches in sarcoma treatment. With pembrolizumab monotherapy showing an 83% progressive disease rate compared to 36% with combination therapy, these findings suggest that immune checkpoint inhibitors alone might have limited efficacy in most sarcoma subtypes. However, there is considerable heterogeneity between the studies (I² = 93.3% for monotherapy and I² = 82.7% for combination therapy), reflecting significant differences in histologic subtypes, patient selection, treatment regimens, and tumor biology. The wide prediction interval (0.00–1.00) indicates uncertainty regarding the reproducibility of these results in future studies. Although this introduces variability, it reflects inherent differences across study populations or methodologies rather than undermining the overall consistency and reliability of the core findings.
The SARC028 trial, evaluating pembrolizumab monotherapy in sarcomas, reported an ORR of 18% in soft tissue sarcomas, with responses primarily observed in undifferentiated pleomorphic sarcoma and liposarcoma [9]. This contrasts with Boye et al’s finding of 0% ORR in a cohort of 12 patients treated with pembrolizumab alone, highlighting the variability in treatment responses across different sarcoma subtypes and patient populations [7]. The limited efficacy of pembrolizumab monotherapy is further supported by the AcSé trial, which reported an ORR of only 6.2% across various rare sarcoma subtypes [18].
The significant variability in treatment outcomes across different histological subtypes underscores the importance of histology-specific approaches in sarcoma management. The current analysis revealed that leiomyosarcoma was the most common histological subtype, yet previous studies have shown this subtype to be relatively resistant to immune checkpoint inhibition. The SARC028 trial reported no responses in leiomyosarcoma patients treated with pembrolizumab monotherapy. In contrast, certain histological subtypes have demonstrated greater sensitivity to immunotherapy. For instance, undifferentiated pleomorphic sarcoma has shown a 40% ORR [9]. Similarly, alveolar soft part sarcoma has emerged as a particularly responsive subtype, with studies reporting response rates of up to 50% with PD-1 inhibition. These findings highlight the critical importance of histology-specific patient selection for immunotherapy trials in sarcoma [19].
The current meta-analysis demonstrated that combination approaches significantly improve treatment outcomes compared to pembrolizumab monotherapy, with combination therapy showing a 64% lower rate of progressive disease. This substantial improvement suggests that combining immune checkpoint inhibitors with other therapeutic modalities may overcome some of the inherent resistance mechanisms in sarcomas.
The combination of pembrolizumab with doxorubicin showed an ORR of 19% [10]. This combination demonstrated manageable toxicity and promising activity. The synergistic effect may be attributed to chemotherapy-induced immunogenic cell death, which can enhance T-cell priming and activation, potentially converting "cold" tumors into "hot" immunogenic ones [20]. The combinations of pembrolizumab with axitinib and lenvatinib showed ORRs of 25% and 15.2%, respectively [11,12]. Wilky et al. reported that axitinib plus pembrolizumab demonstrated manageable toxicity and preliminary activity in advanced sarcomas, particularly in alveolar soft part sarcoma, with a 3-month progression-free survival rate of 65.6% [11]. Similarly, a trial of lenvatinib plus pembrolizumab showed promising activity in certain sarcoma subtypes, including undifferentiated pleomorphic sarcoma and liposarcoma, and malignant peripheral nerve sheath tumors [12]. The enhanced efficacy of these combinations may be attributed to the immunomodulatory effects of tyrosine kinase inhibitors, particularly those targeting VEGF pathways [11].
Novel combination approaches, such as pembrolizumab with talimogene laherparepvec, showed the highest ORR at 35%. Talimogene laherparepvec is an oncolytic virus that can induce immunogenic cell death and enhance systemic anti-tumor immunity, potentially synergizing with immune checkpoint inhibition [13]. In contrast, the combination of pembrolizumab with epacadostat showed an ORR of 3.3%. Epacadostat is an Indoleamine 2,3-dioxygenase 1 inhibitor that was initially thought to complement immune checkpoint inhibition by targeting a different immunosuppressive pathway. However, this combination has shown limited efficacy across multiple tumor types, suggesting that IDO1 inhibition may not be a viable strategy for enhancing immunotherapy responses in sarcomas [14].
The current analysis revealed significant differences in the toxicity profiles between pembrolizumab monotherapy and combination therapy. While combination therapy was associated with better tumor responses, it also resulted in a higher incidence of certain adverse events, particularly thrombocytopenia, hypothyroidism, and hepatobiliary abnormalities. This is consistent with previous studies showing that combining immune checkpoint inhibitors with other therapeutic agents often increases toxicity. A meta-analysis of 18 studies with 2,767 patients found that the risk of severe (grade 3 or higher) adverse events was more than double for combination therapy (risk ratio 2.21, 95% CI 1.57–3.10) [21].
The significant heterogeneity in treatment responses observed in the current analysis and previous studies underscores the need for a personalized approach to sarcoma management. For example, the recent UK guidelines for soft tissue sarcoma management emphasize that certain sarcoma subtypes, such as Ewing sarcoma and rhabdomyosarcoma, require distinct treatment approaches [22].
While the current analysis showed improved outcomes with combination therapy compared to pembrolizumab monotherapy, there is still significant room for improvement. Novel combination strategies targeting multiple aspects of tumor biology and the immune microenvironment may further enhance treatment outcomes [19]. Combinations of immune checkpoint inhibitors with radiation therapy have shown promise in preclinical studies and early clinical trials. Radiation can induce immunogenic cell death, increase neoantigen presentation, and enhance T-cell infiltration, potentially synergizing with immune checkpoint inhibition [19]. Despite initial responses, many patients eventually develop resistance to immunotherapy. Understanding the mechanisms of primary and acquired resistance is crucial for developing effective strategies to overcome resistance. Potential mechanisms include loss of tumor antigens, upregulation of alternative immune checkpoints, and recruitment of immunosuppressive cells to the tumor microenvironment [23].
Conclusion
Pembrolizumab-based combination therapies have the potential to enhance treatment efficacy in sarcoma, although they may be associated with an increased risk of adverse events.
Declarations
Conflicts of interest: The authors have no conflicts of interest to disclose.
Ethical approval: Not applicable.
Consent for participation: Not applicable.
Consent for publication: Not applicable.
Funding: The present study received no financial support.
Acknowledgements: None to be declared.
Authors' contributions: RMA, SSO, SHA, SRH and MMA: major contributors to the conception of the study, as well as the literature search for related studies, and manuscript writing. FHF, HMH, AMM, SOQ, YMM and ADA: Literature review, design of the study, critical revision of the manuscript, and processing of the tables. AGH, DOQ, and MMA: Literature review, processing of the figures, data analysis and interpretation. All authors have read and approved the final version of the manuscript.
Use of AI: ChatGPT-3.5 was used to assist with language refinement and improve the overall clarity of the manuscript. All content was thoroughly reviewed and approved by the authors, who bear full responsibility for the final version.
Data availability statement: Not applicable.
References
- Anastasiou M, Kyriazoglou A, Kotsantis I, Economopoulou P, Kyrkasiadou M, Giannopoulou A et al. Immune checkpoint inhibitors in sarcomas: a systematic review. Immuno-Oncology and Technology. 2023;20:100407. doi:10.1016/j.iotech.2023.100407
- Ahuja S, Zaheer S. The evolution of cancer immunotherapy: a comprehensive review of its history and current perspectives. Korean Journal of Clinical Oncology. 2024;20(2):51. doi:10.14216/kjco.24009
- Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nature reviews Drug discovery. 2019(3):175-96. doi:10.1038/s41573-018-0006-z
- Koury J, Lucero M, Cato C, Chang L, Geiger J, Henry D et al. Immunotherapies: exploiting the immune system for cancer treatment. Journal of immunology research. 2018;2018(1):9585614. doi:10.1155/2018/9585614
- Lee CH, Shah AY, Rasco D, Rao A, Taylor MH, Di Simone C et al. Lenvatinib plus pembrolizumab in patients with either treatment-naive or previously treated metastatic renal cell carcinoma (Study 111/KEYNOTE-146): a phase 1b/2 study. The lancet oncology. 2021;22(7):946-58. doi:10.1016/S1470-2045(21)00241-2
- Sul J, Blumenthal GM, Jiang X, He K, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of patients with metastatic non-small cell lung cancer whose tumors express programmed death-ligand 1. The oncologist. 2016;21(5):643-50. doi:10.1634/theoncologist.2015-0498
- Boye K, Longhi A, Guren T, Lorenz S, Næss S, Pierini M et al. Pembrolizumab in advanced osteosarcoma: results of a single-arm, open-label, phase 2 trial. Cancer Immunology, Immunotherapy. 2021;70:2617-24. doi:10.1007/s00262-021-02876-w
- Hiwa O. Abdullah, Berun A. Abdalla, Fahmi H. Kakamad, Jafaar O. Ahmed, Hiwa O. Baba, Marwan N. Hassan, et al. Predatory Publishing Lists: A Review on the Ongoing Battle Against Fraudulent Actions. Barw Medical Journal. 2024;2(3). doi:10.58742/bmj.v2i2.91
- Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J et al. Pembrolizumab in advanced soft tissue and bone sarcomas: results of SARC028, a multicentre, single arm, phase 2 trial. The Lancet. Oncology. 2017;18(11):1493. doi:10.1016/S1470-2045(17)30624-1
- Pollack SM, Redman MW, Baker KK, Wagner MJ, Schroeder BA, Loggers ET et al. Assessment of doxorubicin and pembrolizumab in patients with advanced anthracycline-naive sarcoma: a phase 1/2 nonrandomized clinical trial. JAMA oncology. 2020;6(11):1778-82. doi:10.1001/jamaoncol.2020.3689
- Wilky BA, Trucco MM, Subhawong TK, Florou V, Park W, Kwon D et al. Axitinib plus pembrolizumab in patients with advanced sarcomas including alveolar soft-part sarcoma: a single-centre, single-arm, phase 2 trial. The lancet oncology. 2019;20(6):837-48. doi:10.1016/S1470-2045(19)30153-6
- Movva S, Seier K, Avutu V, Banks LB, Chan J, Chi P et al. Histology-specific clinical trial of lenvatinib and pembrolizumab in patients with sarcoma. Clinical Cancer Research. 2024;30(24):5612-9. doi:10.1158/1078-0432.CCR-24-2519
- Kelly CM, Antonescu CR, Bowler T, Munhoz R, Chi P, Dickson MA et al. Objective response rate among patients with locally advanced or metastatic sarcoma treated with talimogene laherparepvec in combination with pembrolizumab: a phase 2 clinical trial. JAMA oncology. 2020;6(3):402-8. doi:10.1001/jamaoncol.2019.6152
- Kelly CM, Qin LX, Whiting KA, Richards AL, Avutu V, Chan JE et al. A phase II study of epacadostat and pembrolizumab in patients with advanced sarcoma. Clinical Cancer Research. 2023;29(11):2043-51. doi:10.1158/1078-0432.CCR-22-3911
- Toulmonde M, Penel N, Adam J, Chevreau C, Blay JY, Le Cesne A et al. Use of PD-1 targeting, macrophage infiltration, and IDO pathway activation in sarcomas: a phase 2 clinical trial. JAMA oncology. 2018;4(1):93-7. doi:10.1001/jamaoncol.2017.1617
- Schöffski P, Bahleda R, Wagner AJ, Burgess MA, Junker N, Chisamore M et al. Results of an open-label, phase Ia/b study of pembrolizumab plus olaratumab in patients with unresectable, locally advanced, or metastatic soft-tissue sarcoma. Clinical Cancer Research. 2023;29(17):3320-8. doi:10.1158/1078-0432.CCR-23-0742
- Haddox CL, Nathenson MJ, Mazzola E, Lin JR, Baginska J, Nau A et al. Phase II study of eribulin plus pembrolizumab in metastatic soft-tissue sarcomas: clinical outcomes and biological correlates. Clinical Cancer Research. 2024;30(7):1281-92.
- Blay JY, Chevret S, Le Cesne A, Brahmi M, Penel N, Cousin S et al. Pembrolizumab in patients with rare and ultra-rare sarcomas (AcSé Pembrolizumab): analysis of a subgroup from a non-randomised, open-label, phase 2, basket trial. The Lancet Oncology. 2023;24(8):892-902. doi:10.1016/S1470-2045(23)00282-6
- Wood GE, Meyer C, Petitprez F, D'Angelo SP. Immunotherapy in sarcoma: current data and promising strategies. American Society of Clinical Oncology Educational Book. 2024;44(3):e432234. doi:10.1200/EDBK_432234
- Vanmeerbeek I, Sprooten J, De Ruysscher D, Tejpar S, Vandenberghe P, Fucikova J et al. Trial watch: chemotherapy-induced immunogenic cell death in immuno-oncology. Oncoimmunology. 2020;9(1):1703449. doi:10.1080/2162402X.2019.1703449
- Park R, Lopes L, Cristancho CR, Riano IM, Saeed A. Treatment-related adverse events of combination immune checkpoint inhibitors: systematic review and meta-analysis. Frontiers in oncology. 2020;10:258. doi:10.3389/fonc.2020.00258
- Hayes AJ, Nixon IF, Strauss DC, Seddon BM, Desai A, Benson C et al. UK guidelines for the management of soft tissue sarcomas. British journal of cancer. 2025;132(1):11-31. doi:10.1038/s41416-024-02674-y
- Huang H, Fan Y, Zhang S, Bai X, Wang X, Shan F. Emerging immunotherapy and tumor microenvironment for advanced sarcoma: a comprehensive review. Frontiers in Immunology. 2025;16:1507870. doi:10.3389/fimmu.2025.1507870
Copyright (c) 2025 The Author(s)

This work is licensed under a Creative Commons Attribution 4.0 International License.



